11460
8-Azaxanthine monohydrate
≥98.0% (HPLC)
Synonym(s):
2,6-Dihydroxy-8-azapurine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H3N5O2 · H2O
CAS Number:
Molecular Weight:
171.11
Beilstein:
10424
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
form
solid
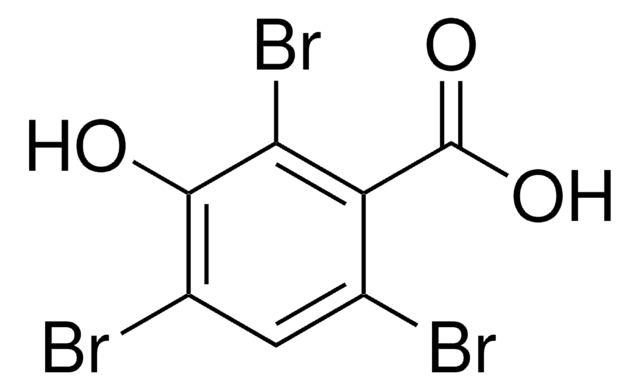
SMILES string
O.O=C1NC(=O)c2[nH]nnc2N1
InChI
1S/C4H3N5O2.H2O/c10-3-1-2(8-9-7-1)5-4(11)6-3;/h(H3,5,6,7,8,9,10,11);1H2
InChI key
VKEGPGRANAWNIN-UHFFFAOYSA-N
Application
8-Azaxanthine monohydrate has been used to determine the crystal and molecular structure of 1,3-dimethyl-8-azaxanthine (HDAX) monohydrate by X-ray diffraction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pascal Retailleau et al.
Acta crystallographica. Section D, Biological crystallography, 60(Pt 3), 453-462 (2004-03-03)
High-resolution X-ray structures of the complexes of Aspergillus flavus urate oxidase (Uox) with three inhibitors, 8-azaxanthin (AZA), 9-methyl uric acid (MUA) and oxonic acid (OXC), were determined in an orthorhombic space group (I222). In addition, the ligand-free enzyme was also
Paxton Forgue et al.
Journal of proteome research, 5(8), 1916-1923 (2006-08-08)
We describe a general protocol for using comparative NMR metabolomics data to infer in vivo efficacy, specificity and toxicity of chemical leads within a drug discovery program. The methodology is demonstrated using Aspergillus nidulans to monitor the activity of urate
L P Ribeiro
Biomedica biochimica acta, 47(2), 107-113 (1988-01-01)
Xanthine dehydrogenase (EC 1.2.1.37) activity was determined in the fat body of the Chagas' disease vector Triatoma infestans using a system in which phenazine methosulphate was associated with p-iodonitrotetrazolium violet as electron acceptors for the oxidation of the substrate xanthine.
Monika Budayova-Spano et al.
Acta crystallographica. Section F, Structural biology and crystallization communications, 62(Pt 3), 306-309 (2006-03-03)
Crystallization and preliminary neutron diffraction measurements of rasburicase, a recombinant urate oxidase enzyme expressed by a genetically modified Saccharomyces cerevisiae strain, complexed with a purine-type inhibitor (8-azaxanthin) are reported. Neutron Laue diffraction data were collected to 2.1 A resolution using
Nathalie Colloc'h et al.
Biochimica et biophysica acta, 1764(3), 391-397 (2006-02-16)
We report the three-dimensional structure determined by high-pressure macromolecular crystallography (HPMX) of a 135-kDa homo-tetrameric enzyme, urate oxidase from Aspergillus flavus complexed with its potent inhibitor 8-azaxanthin. Urate oxidase crystals are quite sensitive to pressure, as three-dimensional order is lost
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service