选择尺寸
25 G
$90.40
100 G
$248.00
250 G
$416.00
1 KG
$1,190.00
$90.40
请联系客服了解存货情况
新价格,新优惠!
所有图片(5)
选择尺寸
变更视图
25 G
$90.40
100 G
$248.00
250 G
$416.00
1 KG
$1,190.00
About This Item
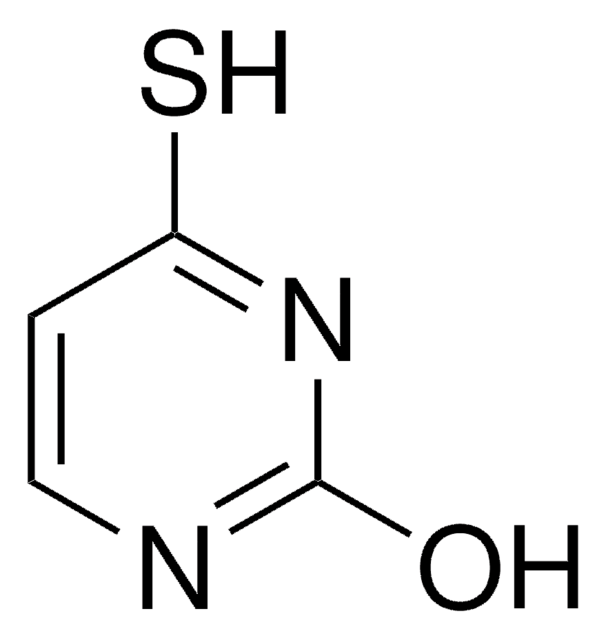
经验公式(希尔记法):
C4H4N2OS
CAS号:
分子量:
128.15
Beilstein:
112227
EC 号:
MDL编号:
UNSPSC代码:
41106305
PubChem化学物质编号:
NACRES:
NA.51
$90.40
请联系客服了解存货情况
新价格,新优惠!
推荐产品
生物来源
synthetic
方案
≥99%
表单
powder
mp
>300 °C (lit.)
溶解性
1 M NaOH: 5 mg/mL, clear to very slightly hazy
1 M NaOH: 50 mg/mL, colorless to faintly yellow
SMILES字符串
O=C1NC(=S)NC=C1
InChI
1S/C4H4N2OS/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8)
InChI key
ZEMGGZBWXRYJHK-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
应用
警示用语:
Warning
危险声明
危险分类
Carc. 2
储存分类代码
11 - Combustible Solids
WGK
WGK 3
个人防护装备
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
A Palumbo et al.
FEBS letters, 485(2-3), 109-112 (2000-11-30)
2-thiouracil (TU), an established antithyroid drug and melanoma-seeker, was found to selectively inhibit neuronal nitric oxide synthase (nNOS) in a competitive manner (K(i)=20 microM), being inactive on the other NOS isoforms. The drug apparently interfered with the substrate- and tetrahydrobiopterin
Silver colloid and film substrates in surface-enhanced Raman scattering for 2-thiouracil detection
Al-Shalalfeh MM, et al.
Royal Society of Chemistry Advances, 6, 75282-75292 (2016)
Leslie Gay et al.
Genes & development, 27(1), 98-115 (2013-01-12)
Transcriptional profiling is a powerful approach for understanding development and disease. Current cell type-specific RNA purification methods have limitations, including cell dissociation trauma or inability to identify all RNA species. Here, we describe "mouse thiouracil (TU) tagging," a genetic and
J Vanden Bussche et al.
Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment, 28(2), 166-172 (2011-01-26)
Thiouracil belongs to the xenobiotic thyreostats, which are growth-promoting agents illegally used in animal production. Recently it has been reported that thiouracil is suspected to have a natural origin. The European Union of Reference Laboratory guidance paper of 2007 acknowledged
Raj Kumar Bera et al.
Chemical communications (Cambridge, England), 47(41), 11498-11500 (2011-09-22)
A nonenzymatic method for the selective detection and quantification of serum uric acid (UA) using 2-thiouracil (2-TU) tailored Au nanoparticles is developed. The H-bonding interaction of UA with functionalized Au nanoparticles brings instantaneous visible color change and paves the way
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门