About This Item
推荐产品
质量水平
方案
≥98% (HPLC)
表单
solid
储存条件
desiccated
under inert gas
颜色
white
溶解性
DMSO: >20 mg/mL
H2O: ≥5 mg/mL
创始人
AstraZeneca
储存温度
2-8°C
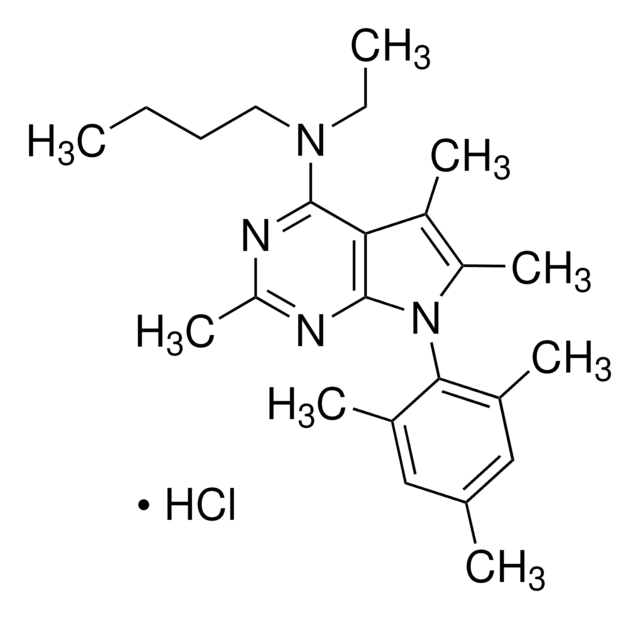
SMILES字符串
Cl[H].CC(N)C(=O)Nc1c(C)cccc1C
InChI
1S/C11H16N2O.ClH/c1-7-5-4-6-8(2)10(7)13-11(14)9(3)12;/h4-6,9H,12H2,1-3H3,(H,13,14);1H
InChI key
AMZACPWEJDQXGW-UHFFFAOYSA-N
基因信息
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
正在寻找类似产品? 访问 产品对比指南
应用
生化/生理作用
特点和优势
警示用语:
Warning
危险声明
危险分类
Acute Tox. 4 Oral
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
商品
电压门控钠通道存在于大多数可兴奋细胞膜中,在产生动作电位方面起着重要作用。
Voltage-gated sodium channels are present in most excitable cell membranes and play an important role in generating action potentials.
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门