SML0259
长春质碱
≥95% (HPLC)
别名:
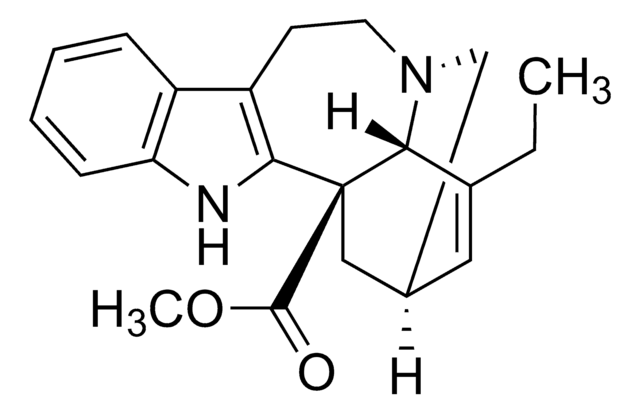
(+)-3,4-Didehydrocoronaridine, (2α,5β,6α,18β)-3,4-Didehydroibogamine-18-carboxylic acid methyl ester, 7-Ethyl-9,10,12,13-tetrahydro-6,9-methano-5H-pyrido[1′,2′:1,2]azepino[4,5-b]indole-6(6aH)-carboxylic acid methyl ester
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C21H24N2O2
CAS号:
分子量:
336.43
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推荐产品
化驗
≥95% (HPLC)
形狀
powder
光學活性
[α]/D +30 to +38° (c=0.5, CDCl3)
顏色
white to beige
溶解度
DMSO: 5 mg/mL (clear solution; warmed)
運輸包裝
wet ice
儲存溫度
−20°C
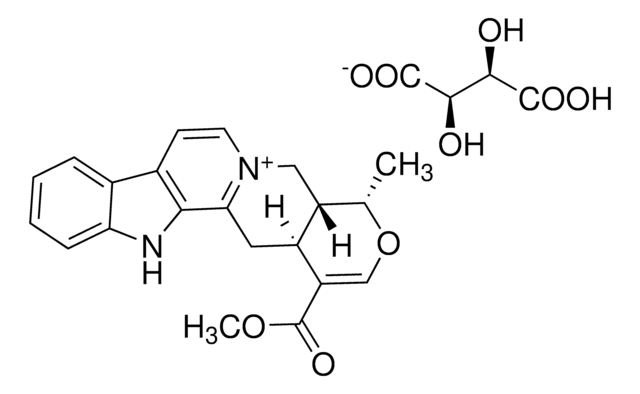
SMILES 字串
CCC1=C[C@@H]2C[N@H]3CCc4c([nH]c5ccccc45)[C@@](C2)([C@@H]13)C(=O)OC
InChI
1S/C21H24N2O2/c1-3-14-10-13-11-21(20(24)25-2)18-16(8-9-23(12-13)19(14)21)15-6-4-5-7-17(15)22-18/h4-7,10,13,19,22H,3,8-9,11-12H2,1-2H3/t13-,19+,21-/m0/s1
InChI 密鑰
CMKFQVZJOWHHDV-NQZBTDCJSA-N
正在寻找类似产品? 访问 产品对比指南
生化/生理作用
Catharanthine is a precursor of the anti-tumor drugs vinblastine and vincristine, formed by dimerization of catharanthine with vindoline. Catharanthine itself is an inhibitor of tubulin self-assembly into microtubules, although not so potent as vinblastine or vincristine. Catharanthine also has anticholinergic activity. It showed muscarinic antagonism at 10 microM and fully inhibited nicotinic receptor mediated diaphragm contractions with an IC50 of 59.6 microM.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Hugo R Arias et al.
Neurochemistry international, 57(2), 153-161 (2010-05-25)
We compared the interaction of several catharanthine alkaloids including, ibogaine, vincristine, and vinblastine, with that for the noncompetitive antagonist phencyclidine (PCP) at muscle nicotinic acetylcholine receptors (AChRs) in different conformational states. The results established that catharanthine alkaloids: (a) inhibit, in
Hiroaki Gotoh et al.
Journal of the American Chemical Society, 134(32), 13240-13243 (2012-08-04)
A definition of the scope of aromatic substrates that participate with catharanthine in an Fe(III)-mediated coupling reaction, an examination of the key structural features of catharanthine required for participation in the reaction, and the development of a generalized indole functionalization
Mei-Liang Zhou et al.
Applied microbiology and biotechnology, 88(3), 737-750 (2010-08-18)
Jasmonates and nitric oxide (NO) play important roles in the regulation of the signaling network leading to the biosynthesis of plant secondary metabolites. In this work, we explore the effect of constitutive overexpression of CrORCA3 (octadecanoid-responsive Catharanthus AP2/ERF domain), methyl
Cui-Ting Wang et al.
Plant cell reports, 29(8), 887-894 (2010-06-11)
A number of genes that function in the terpenoid indole alkaloids (TIAs) biosynthesis pathway have been identified in Catharanthus roseus. Except for the geraniol 10-hydroxylase (G10H) gene, which encodes a cytochrome P450 monooxygenase, several of these genes are up-regulated by
Annie Tam et al.
Bioorganic & medicinal chemistry letters, 20(22), 6408-6410 (2010-10-12)
The examination of the catharanthine C16 substituent effects on the Fe(III)-promoted biomimetic coupling reaction with vindoline is detailed, confirming the importance of the presence of a C16 electron-withdrawing substituent, and establishing an unanticipated unique role (>10-fold) that the C16 methyl
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门