推荐产品
品質等級
化驗
≥98%
形狀
powder
儲存條件
(Keep container tightly closed in a dry and well-ventilated place.)
顏色
white to off-white
溶解度
H2O: soluble 50 mg/mL
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
作用方式
DNA synthesis | interferes
enzyme | inhibits
儲存溫度
2-8°C
SMILES 字串
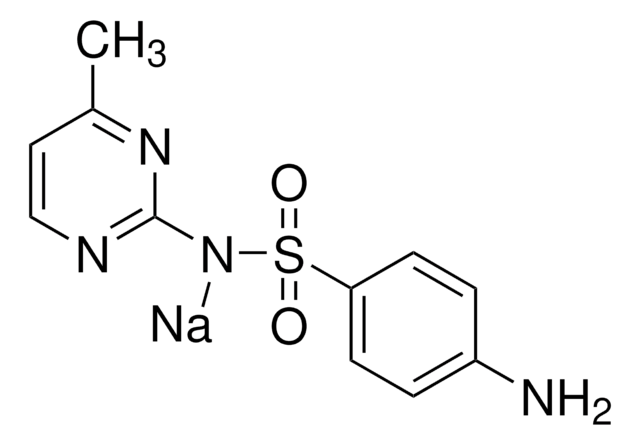
[Na].Cc1cc(C)nc(NS(=O)(=O)c2ccc(N)cc2)n1
InChI
1S/C12H14N4O2S.Na.H/c1-8-7-9(2)15-12(14-8)16-19(17,18)11-5-3-10(13)4-6-11;;/h3-7H,13H2,1-2H3,(H,14,15,16);;
InChI 密鑰
WIVZAHIZHZEEOX-UHFFFAOYSA-N
相关类别
一般說明
化学结构:磺胺
應用
磺胺二甲嘧啶是一种抗生素,在临床上用于治疗支气管炎、前列腺炎和尿路感染。用于处置和消除动力学研究。其用于开发液体(如牛奶、蜂蜜和猪尿)中的定量检测技术。
生化/生理作用
磺胺二甲嘧啶是一种抗微生物性硫药,通过抑制二氢喋呤合成酶而阻断二氢叶酸的合成。磺胺二甲嘧啶是细菌合成叶酸所需的对氨基苯甲酸 (PABA) 的竞争性抑制剂。诱导 CYP3A4 表达并被 N-乙酰转移酶乙酰化。它表现出性别依赖性药代动力学,由男性特有的 CYP2C11 亚型代谢。磺胺二甲嘧啶具有抑菌作用。
包裝
25G,100G
其他說明
保存于密闭容器内,置于干燥通风处。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Bożena Czubat et al.
Frontiers in microbiology, 11, 2008-2008 (2020-09-26)
MSMEG_4305 is a two-domain protein of Mycolicibacterium smegmatis (Mycobacterium smegmatis) (Mycolicibacterium smegmatis). The N-terminal domain of MSMEG_4305 encodes an RNase H type I. The C-terminal domain is a presumed CobC, predicted to be involved in the aerobic synthesis of vitamin
Tangbin Yang et al.
Hybridoma (2005), 29(5), 403-407 (2010-11-06)
A specific monoclonal antibody (MAb) against sulfamethazine was produced with hybridoma technology. This assay shows very high sensitivity with IC50 of 0.4 ng/mL and LOD of 0.05 ng/mL when it was run in 0.02 mol/L PBS (pH 7.5). This MAb has shown high
C J Chapron et al.
Journal of clinical pharmacology, 16(7), 338-344 (1976-07-01)
The relationship between sulfamethazine disposition kinetics and acetylation phenotype was studied in man. Sulfamethazine pharmacokinetic parameters were determined after the administration of the drug as an oral suspension. When the half-life, acetylation rate constant, or per cent available dose excreted
A D Mitchell et al.
Drug metabolism and disposition: the biological fate of chemicals, 14(2), 161-165 (1986-03-01)
Swine weighing 60-70 kg were orally administered 14C-sulfamethazine [4-amino-N-(4,6-dimethyl-2-pyrimidinyl)benzene[U-14C]sulfonamide] at 12-hr intervals for 7 days (165 mg/dose; 0.126-5.04 mCi/mmol). The animals were sacrificed at 8 hr or 2, 5, or 10 days after the last dose was given and tissues
Craig Knox et al.
Nucleic acids research, 39(Database issue), D1035-D1041 (2010-11-10)
DrugBank (http://www.drugbank.ca) is a richly annotated database of drug and drug target information. It contains extensive data on the nomenclature, ontology, chemistry, structure, function, action, pharmacology, pharmacokinetics, metabolism and pharmaceutical properties of both small molecule and large molecule (biotech) drugs.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门