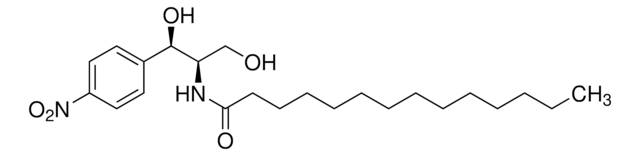
About This Item
推荐产品
product name
Reveromycin A, solid
生物源
Streptomyces sp.
品質等級
形狀
solid
分子量
660.79 g/mol
包裝
pkg of 100 μg
顏色
tan
溶解度
DMF: soluble
DMSO: soluble
ethanol: soluble
ethyl acetate: soluble
methanol: soluble
soluble
抗生素活性譜
fungi
作用方式
protein synthesis | interferes
運輸包裝
wet ice
儲存溫度
−20°C
SMILES 字串
[H][C@]1(C\C=C(C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(C)=C\C(O)=O
InChI
1S/C36H52O11/c1-6-7-19-35(47-34(44)17-16-32(40)41)21-22-36(46-30(35)14-10-25(3)23-33(42)43)20-18-27(5)29(45-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-12,14-15,23,26-30,37H,6-7,13,16-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,15-11+,24-9+,25-23+/t26-,27-,28-,29+,30-,35+,36-/m0/s1
InChI 密鑰
ZESGNAJSBDILTB-OXVOKJAASA-N
生化/生理作用
分析報告
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门