推荐产品
product name
Z-Phe-Arg 7-酰氨基-4-甲基香豆素 盐酸盐, kallikrein substrate
化驗
≥95% (HPLC)
形狀
powder
濃度
≥95%
溶解度
methanol: 20 mg/mL, clear, colorless
儲存溫度
−20°C
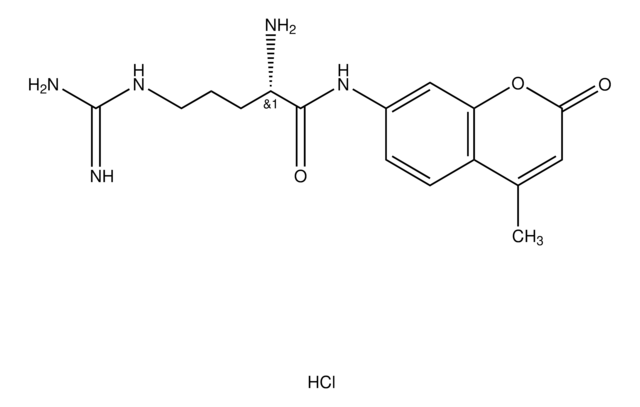
SMILES 字串
O=C(N[C@@H](CC1=CC=CC=C1)C(N[C@@H](CCCNC(N)=N)C(NC2=CC=C(C(C)=CC(O3)=O)C3=C2)=O)=O)OCC4=CC=CC=C4.[Cl]
InChI
1S/C33H36N6O6/c1-21-17-29(40)45-28-19-24(14-15-25(21)28)37-30(41)26(13-8-16-36-32(34)35)38-31(42)27(18-22-9-4-2-5-10-22)39-33(43)44-20-23-11-6-3-7-12-23/h2-7,9-12,14-15,17,19,26-27H,8,13,16,18,20H2,1H3,(H,37,41)(H,38,42)(H,39,43)(H4,34,35,36)
InChI 密鑰
ZZGDDBWFXDMARY-UHFFFAOYSA-N
一般說明
Z-苯丙氨酸-精氨酸 7-氨基-4-甲基香豆素(Z-FR-AMC)是一种拟肽类底物,可用于木瓜蛋白酶和其它酶,如组织蛋白酶K。它也是组织蛋白酶L和B的荧光合成肽。
應用
Z-苯丙氨酸-精氨酸7-氨基-4-甲基香豆盐酸盐已被用于:
- 作为猕猴桃碱抑制检测中的荧光底物
- 作为血管舒缓素底物
- 作为荧光检测的胰蛋白酶底物
- 作为组织蛋白酶-L底物
生化/生理作用
由蛋白酶引发的Z-苯丙氨酸-精氨酸7-氨基-4-甲基香豆素(Z-FR-AMC)的蛋白水解性裂解导致AMC的释放,结果使酶反应中的荧光增强。
基底
一种用于血浆激肽释放酶的荧光底物。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
H Ghoneim et al.
International journal for parasitology, 25(12), 1515-1519 (1995-12-01)
A previously described "major acidic proteinase" of adult Schistosoma mansoni, believed to play a key role in the parasite's metabolism, has been identified as a cathepsin B (Sm31). Purified Sm cathepsin B was not recognized by anti-Sm32 or anti-cathepsin L
Fabien Lecaille et al.
The Biochemical journal, 375(Pt 2), 307-312 (2003-07-03)
The limited availability of highly selective cathepsin substrates seriously impairs studies designed to monitor individual cathepsin activities in biological samples. Among mammalian cysteine proteases, cathepsin K has a unique preference for a proline residue at P2, the primary determinant of
P J Rosenthal
Experimental parasitology, 80(2), 272-281 (1995-03-01)
The effects of peptide proteinase inhibitors on globin hydrolysis by cultured malaria parasites were studied. All of the four cysteine proteinase inhibitors evaluated blocked globin hydrolysis, as documented by the development of a morphological abnormality in which parasite food vacuoles
C Illy et al.
The Journal of biological chemistry, 272(2), 1197-1202 (1997-01-10)
Within the lysosomal cysteine protease family, cathepsin B is unique due to its ability to act both as an endopeptidase and a peptidyldipeptidase. This latter capacity to remove C-terminal dipeptides has been attributed to the presence of a 20-residue insertion
R M C Deshapriya et al.
Journal of biochemistry, 147(3), 393-404 (2009-11-17)
To identify functionally essential sequences and residues of CTLA-2alpha, in vitro mutagenesis was carried out. The coefficient of inhibition (K(i)) was determined towards rabbit cathepsin L using Z-Phe-Arg-MCA as the substrate. Recombinant CTLA-2alpha inhibited the enzyme potently (K(i) = 15
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门