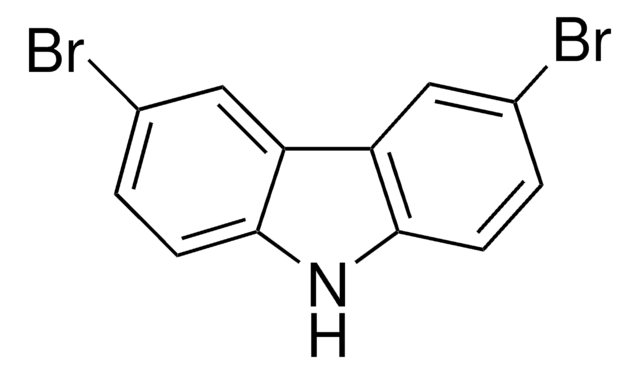
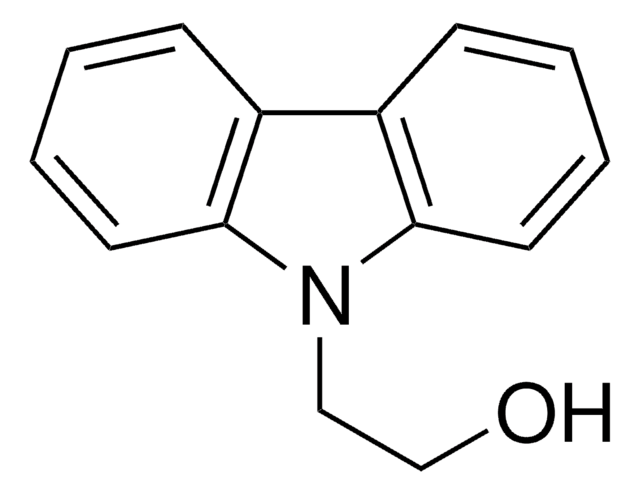
推荐产品
product name
咔唑, ≥95% (GC)
蒸汽壓力
400 mmHg ( 323 °C)
品質等級
化驗
≥95% (GC)
形狀
powder
顏色
white to tan
bp
355 °C (lit.)
mp
243-246 °C (lit.)
溶解度
acetone: 50 mg/mL
密度
1.1 g/cm3 at 18 °C
應用
diagnostic assay manufacturing
hematology
histology
儲存溫度
room temp
SMILES 字串
c1ccc2c(c1)[nH]c3ccccc23
InChI
1S/C12H9N/c1-3-7-11-9(5-1)10-6-2-4-8-12(10)13-11/h1-8,13H
InChI 密鑰
UJOBWOGCFQCDNV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
咔唑是一种芳香族杂环有机分子。
應用
咔唑已用于糖醛酸的测定。
生化/生理作用
咔唑主要与 DNA 相互作用并对其造成损伤。这些事件可抑制新 DNA 或 RNA 的合成。咔唑衍生物具有抗微生物、抗肿瘤、抗癫痫、抗组胺、抗氧化、抗炎、抗腹泻、镇痛、神经保护和胰脂肪酶抑制活性。
品質
其可能含有黑色颗粒物。
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 4 - Carc. 2 - Muta. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
428.0 °F - closed cup
閃點(°C)
220.0 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
CARBAZOLE DERIVATIVES IN CANCER TREATMENT-A REVIEW.
Kanneti Naga M, et al.
World Journal of Pharmacy and Pharmaceutical Sciences (2015)
A complete set of hyaluronan fragments obtained from hydrolysis catalyzed by hyaluronidase: Application to studies of hyaluronan mass distribution by simple HPLC devices.
Tranchepain F, et al.
Analytical Biochemistry, 348, 232-242 (2006)
Yuji Ashikawa et al.
BMC structural biology, 12, 15-15 (2012-06-26)
Dihydroxylation of tandemly linked aromatic carbons in a cis-configuration, catalyzed by multicomponent oxygenase systems known as Rieske nonheme iron oxygenase systems (ROs), often constitute the initial step of aerobic degradation pathways for various aromatic compounds. Because such RO reactions inherently
Guodong Ji et al.
Journal of hazardous materials, 225-226, 1-7 (2012-05-23)
We examined the effects of power and treatment time on the ultrasonically enhanced ozonation of carbazole dissolved in APG(1214) surfactant solutions, including an analysis of the mechanism of OH radical formation, the zeta potential of the colloidal suspension, the influence
Shen H Tan et al.
Organic letters, 14(22), 5621-5623 (2012-10-31)
The racemic modification of the Aspidosperma alkaloid limaspermidine (1) has been prepared in ten steps including one involving a Raney-cobalt-mediated tandem reductive cyclization of nitrile 8 to give the tetracyclic system 9b. Compound (±)-1 has been converted over two steps
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门