推荐产品
化驗
≥98% (HPLC)
形狀
powder
顏色
white to tan
溶解度
DMSO: ≥18 mg/mL
起源
Merck & Co., Inc., Kenilworth, NJ, U.S.
儲存溫度
2-8°C
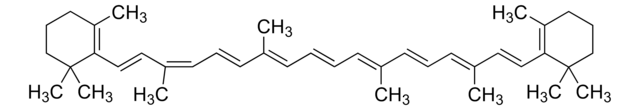
SMILES 字串
Cc1c(C)c2C(=O)C(Cc2cc1OCc3cccc(c3)-c4ccc(cc4)C(O)=O)C5CCCC5
InChI
1S/C30H30O4/c1-18-19(2)28-25(15-26(29(28)31)22-7-3-4-8-22)16-27(18)34-17-20-6-5-9-24(14-20)21-10-12-23(13-11-21)30(32)33/h5-6,9-14,16,22,26H,3-4,7-8,15,17H2,1-2H3,(H,32,33)
InChI 密鑰
KMKBEESNZAPKMP-UHFFFAOYSA-N
生化/生理作用
Biphenyl-indanone A (BINA) is a potent selective positive allosteric modulator for the group II metabotropic glutamate receptor subtype mGluR2. In animal studies BINA showed anxiolytic and antipsychotic effects, and blocked the effects produced by the hallucinogenic drug DOB. It decreased cocaine self-administration in rats, with no effect on food self-administration. In recombinant systems, BINA selectively potentiated the response of mGluR2 to glutamate with no effect on the glutamate response of other mGluR receptor subtypes tested.
特點和優勢
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Glutamate Receptors (G Protein Family) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Merck & Co., Inc., Kenilworth, NJ, U.S.. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jun-Tao Gao et al.
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 43(13), 2615-2626 (2018-10-05)
Opioid abuse is a rapidly growing public health crisis in the USA. Despite extensive research in the past decades, little is known about the etiology of opioid addiction or the neurobiological risk factors that increase vulnerability to opioid use and
Thor C Møller et al.
Scientific reports, 8(1), 10414-10414 (2018-07-12)
G protein coupled receptors (GPCRs) play essential roles in intercellular communication. Although reported two decades ago, the assembly of GPCRs into dimer and larger oligomers in their native environment is still a matter of intense debate. Here, using number and
Guendalina Olivero et al.
British journal of pharmacology, 174(24), 4785-4796 (2017-10-03)
We recently proposed the existence of mGlu We studied the effect of LY566332, an mGlu Cortical synaptosomes possess LY566332-sensitive autoreceptors that are slightly, although significantly, susceptible to LY2389575. In contrast, LY566332-insensitive and LY2389575-sensitive autoreceptors are present in spinal cord terminals.
Maarten L J Doornbos et al.
Biochemical pharmacology, 155, 356-365 (2018-07-22)
While many orthosteric ligands have been developed for the mGlu2 receptor, little is known about their target binding kinetics and how these relate to those of the endogenous agonist glutamate. Here, the kinetic rate constants, i.e. kon and koff, of
商品
Sigma-Aldrich offers many products related to G-protein family glutamate receptors for your research needs.
DISCOVER Bioactive Small Molecules for Neuroscience
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门