所有图片(1)
选择尺寸
变更视图
5 MG
$150.00
25 MG
$590.00
About This Item
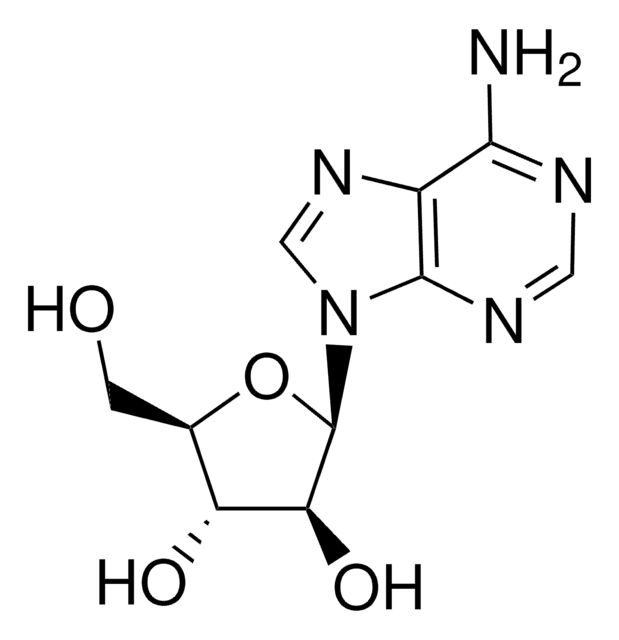
经验公式(希尔记法):
C10H13N5O5 · xH2O
CAS号:
分子量:
283.24 (anhydrous basis)
MDL编号:
UNSPSC代码:
12352200
PubChem化学物质编号:
NACRES:
NA.77
推荐产品
方案
≥98% (HPLC)
表单
solid
溶解性
DMSO: >10 mg/mL
H2O: insoluble
储存温度
2-8°C
SMILES字符串
NC1=Nc2c(ncn2[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)C(=O)N1
InChI
1S/C10H13N5O5/c11-10-13-7-4(8(19)14-10)12-2-15(7)9-6(18)5(17)3(1-16)20-9/h2-3,5-6,9,16-18H,1H2,(H3,11,13,14,19)/t3-,5-,6+,9-/m1/s1
InChI key
NYHBQMYGNKIUIF-FJFJXFQQSA-N
应用
Ara-G is converted by cellular kinases to the active 5′-triphosphate, Ara-GTP. This active form of Ara-G induces apoptosis and inhibits DNA synthesis. Ara-G is also an antineoplastic and antimetabolite.
生化/生理作用
Ara-G is an inducer of apoptosis; inhibitor of DNA synthesis; antineoplastic; and antimetabolite.
Ara-G is an inducer of apoptosis; inhibitor of DNA synthesis; antineoplastic; and antimetabolite. Ara-G is converted by cellular kinases to the active 5′-triphosphate, Ara-GTP. Incorporation of Ara-GTP into DNA leads to inhibition of DNA synthesis and apoptosis.
警示用语:
Warning
危险分类
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
A Fyrberg et al.
Biochemical and biophysical research communications, 427(3), 456-460 (2012-08-23)
Our previous data from a human leukemic cell line made resistant to the nucleoside analog (NA) 9-β-D-arabinofuranosylguanine (AraG) revealed a massive upregulation of fetal hemoglobin (HbF) genes and the ABCB1 gene coding for the multidrug resistance P-glycoprotein (P-gp). The expression
Irene Homminga et al.
Blood, 118(8), 2184-2190 (2011-07-07)
Forodesine and nelarabine (the pro-drug of ara-G) are 2 nucleoside analogues with promising anti-leukemic activity. To better understand which pediatric patients might benefit from forodesine or nelarabine (ara-G) therapy, we investigated the in vitro sensitivity to these drugs in 96
Sheryl A Flanagan et al.
Biochemical pharmacology, 66(5), 733-737 (2003-09-02)
We studied acceptance of various deoxyguanosine analogues by the unique guanosine preferring nucleoside transport system exhibited by NB4 cells, csg. Indirect assessment of acceptance using transport inhibition assays revealed that both 1-beta-D-arabinofuranosylguanine (ara-G) and 4'-thio-beta-D-xylofuranosylguanine (thio-xyl-G) compete with guanosine for
Anna Fyrberg et al.
Cancer chemotherapy and pharmacology, 68(3), 583-591 (2010-11-27)
To characterize resistance mechanisms to the nucleoside analog 9-β-D-arabinofuranosylguanine (AraG) in the T-cell acute lymphoblastic leukemia cell line MOLT-4 and its AraG-resistant variant. A gene expression microarray analysis was performed, as well as gene expression and enzyme activity measurements of
M Bjerke et al.
Nucleosides, nucleotides & nucleic acids, 27(6), 746-749 (2008-07-05)
Incubation of cells with thymidine (dThd) is known to cause dNTP pool imbalance as well as deletions and depletion of the mtDNA. In order to gain further understanding in the events involved in dThd toxicity over time, H9 cells were
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门