推荐产品
等級
pharmaceutical primary standard
agency
EP Reference Standard
API 家族
gemfibrozil
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
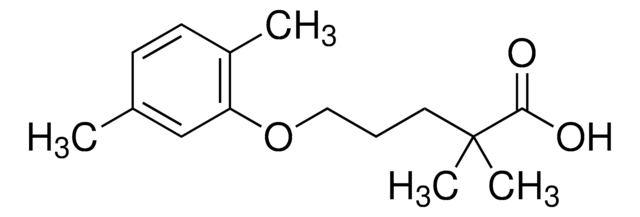
Cc1ccc(C)c(OCCCC(C)(C)C(O)=O)c1
InChI
1S/C15H22O3/c1-11-6-7-12(2)13(10-11)18-9-5-8-15(3,4)14(16)17/h6-7,10H,5,8-9H2,1-4H3,(H,16,17)
InChI 密鑰
HEMJJKBWTPKOJG-UHFFFAOYSA-N
基因資訊
human ... PPARA(5465)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Gemfibrozil EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
A M Filppula et al.
Clinical pharmacology and therapeutics, 94(3), 383-393 (2013-05-10)
Cytochrome P450 (CYP) 3A4 is considered the most important enzyme in imatinib biotransformation. In a randomized, crossover study, 10 healthy subjects were administered gemfibrozil 600 mg or placebo twice daily for 6 days, and imatinib 200 mg on day 3, to study
Ana Maria Sierra Villar et al.
International journal of pharmaceutics, 431(1-2), 161-175 (2012-04-14)
Self-nanoemulsifying drug delivery systems of gemfibrozil were developed under Quality by Design approach for improvement of dissolution and oral absorption. Preliminary screening was performed to select proper components combination. Box-Behnken experimental design was employed as statistical tool to optimize the
Manthena V S Varma et al.
Pharmaceutical research, 29(10), 2860-2873 (2012-05-29)
To develop physiologically based pharmacokinetic (PBPK) model to predict the pharmacokinetics and drug-drug interactions (DDI) of pravastatin, using the in vitro transport parameters. In vitro hepatic sinusoidal active uptake, passive diffusion and canalicular efflux intrinsic clearance values were determined using
P A Todd et al.
Drugs, 36(3), 314-339 (1988-09-01)
Gemfibrozil is a lipid-regulating agent which is generically classified as a fibric acid derivative, but which exhibits different pharmacological effects from other such drugs. Published data indicate that in patients with all types of dyslipidaemia (except type I) gemfibrozil 800
Amir Qaseem et al.
Annals of internal medicine, 159(12), 835-847 (2013-10-23)
The American College of Physicians (ACP) developed this guideline to present the evidence and provide clinical recommendations on the screening, monitoring, and treatment of adults with stage 1 to 3 chronic kidney disease. This guideline is based on a systematic
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门