推荐产品
等级
puriss.
方案
≥99.0% (AT)
灼烧残渣
≤0.05%
mp
175 °C (dec.) (lit.)
175-176 °C (dec.)
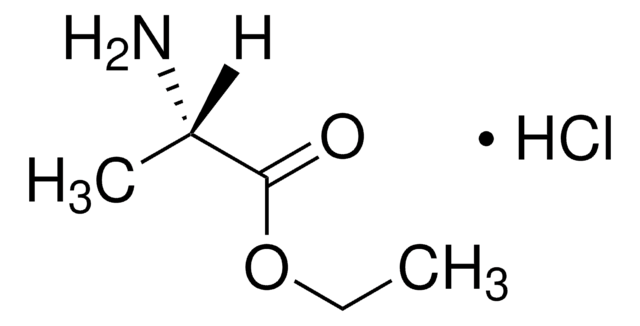
SMILES字符串
Cl.COC(=O)CN
InChI
1S/C3H7NO2.ClH/c1-6-3(5)2-4;/h2,4H2,1H3;1H
InChI key
COQRGFWWJBEXRC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
替代产品
产品编号
说明
价格
M Iwama et al.
Biological & pharmaceutical bulletin, 24(9), 978-981 (2001-09-18)
The sialic acid binding lectin from bullfrog Rana catesbeiana oocyte (cSBL) is known to have anti-tumor activity. In order to investigate the relationship between the net charge of cSBL and its anti-tumor effect, cSBL was modified with a water-soluble carbodiimide
Wenzhong Gao et al.
Organic letters, 7(19), 4241-4244 (2005-09-09)
[reaction: see text] A novel Cu(I)-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides with acrylates has been developed. Up to 98/2 exo/endo selectivity and up to 98% enantiomeric excess have been achieved.
Akihiko Tohri et al.
European journal of biochemistry, 271(5), 962-971 (2004-03-11)
To elucidate the domains on the extrinsic 23 kDa protein involved in electrostatic interaction with the extrinsic 33 kDa protein in spinach photosystem II, we modified amino or carboxyl groups of the 23 kDa protein to uncharged methyl ester groups
J M Delfino et al.
International journal of peptide and protein research, 21(4), 440-450 (1983-04-01)
The reactivity of the carboxyl groups in bovine growth hormone was studied by reaction with 1-ethyl-3(3-dimethylaminopropyl) carbodiimide in the presence of an excess of glycinemethylester. Localization in the molecule of the various kinetically distinguishable carboxyl groups was achieved. Highest reactivity
Vincenzo Santagada et al.
Journal of combinatorial chemistry, 7(4), 618-621 (2005-07-12)
An easy and convenient microwave-assisted synthesis of N-alkylated glycine methyl esters is described. Parallel and nonparallel combinatorial methods are described and compared. The described reactions are reductive alkylations of several aldehydes and glycine methyl ester in the presence of NaBH3CN.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门