5.30588
IκB Ubiquitination Inhibitor, GS143
别名:
IκB Ubiquitination Inhibitor, GS143, 4-(3-Benzyl-4-(5-(2-fluoro-phenyl)-furan-2-ylmethylene)-5-oxo-4,5-dihydropyrazol-1-yl)-benzoic acid, 4-(3-Benzyl-4-((5-(2-fluorophenyl)furan-2-yl)methylene)-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)benzoic acid, GS 143
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
化驗
≥97% (HPLC)
品質等級
形狀
powder
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
dark red
溶解度
DMSO: 25 mg/mL
儲存溫度
2-8°C
SMILES 字串
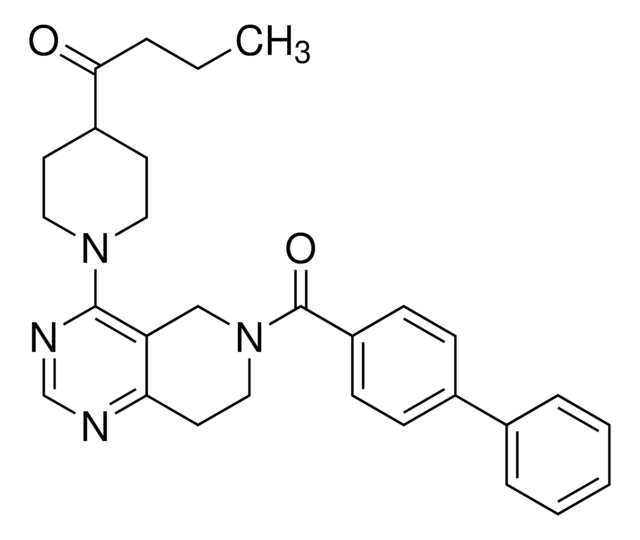
C1=CC=C(C=C1)CC2=NN(C(=O)C2=CC3=CC=C(O3)C4=CC=CC=C4F)C5=CC=C(C=C5)C(=O)O
一般說明
A cell-permeable trisubstituted oxo-dihydropyrazolyl-benzoic acid that selectively inhibits the E3 ligase complex SCFβTrCP1-mediated ubiquitination of phosphorylated IκBα (IC50 = 5.2 µM), displaying little inhibitory potency against IKKβ-catalyzed IκBα phosphorylation, MDM2-mediated p53 ubiquitination, or the protease activities of cathepsin B, chymotrypsin, 20S & 26S proteasome (IC50 >100 µM). Cellular IκBα degradation blockage by GS143 treatment (complete inhibition against TNFα-induced degradation with 30 min 20 µM drug pretreatment in in HeLa S3 and HT29 cultures) effectively inhibits TNFα- and LPS-stimulated NF-κB transcription in HEK293, HT-29, and THP-1 cultures (IC50 from 2.1 to 10.5 µM). Intranasal administration (32 µg/20 µL/mouse) among OVA-sensitized mice 2 h prior to OVA challenge via inhalation greatly suppress OVA challenge-induced NF-κB activation in lung tissue (>90% inhibition of nuclear NF-κB p65 DNA-binding activity 2 h post challenge) and airway inflammation (>80% reduction in eosinophils & lymphocytes in BALF 48 h post challenge) in vivo. GS143 most likely prevents SCFβTrCP1 from interacting with phosphorylated IκBα without inhibiting SCFβTrCP1 E3 ligase activity directly, as no apparent accumulation of another known SCFβTrCP1 substrate β-catenin is seen in HeLa S3 cells upon GS143 treatment (up to 20 µM for 3 h).
A cell-permeable trisubstituted oxo-dihydropyrazolyl-benzoic acid that selectively inhibits the E3 ligase complex SCFβTrCP1-mediated ubiquitination of phosphorylated IκBα (IC50 = 5.2 µM), displaying little inhibitory potency against IKKβ-catalyzed IκBα phosphorylation, MDM2-mediated p53 ubiquitination, or the protease activities of cathepsin B, chymotrypsin, 20S & 26S proteasome (IC50 >100 µM). Effectively inhibits TNFα- and LPS-stimulated NF-κB transcription in HEK293, HT-29, and THP-1 cultures (IC50 from 2.1 to 10.5 µM). Intranasal administration (32 µg/20 µL/mouse) among OVA-sensitized mice 2 h prior to OVA challenge via inhalation greatly suppress NF-κB activation in lung tissue and airway inflammation in vivo. GS143 most likely prevents SCFβTrCP1 from interacting with phosphorylated IκBα without inhibiting SCFβTrCP1 E3 ligase activity directly.
生化/生理作用
Cell permeable: yes
Primary Target
phosphorylated IκBα
phosphorylated IκBα
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Use only fresh DMSO for reconstitution.
其他說明
Hirose, K., et al. 2008. Biochem. Biophys. Res. Commun.374, 507.
Nakajima, H., et al. 2008. Biochem. Biophys. Res. Commun.368, 1007.
Nakajima, H., et al. 2008. Biochem. Biophys. Res. Commun.368, 1007.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门