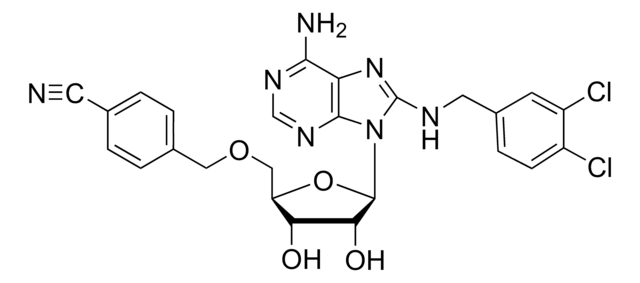
A cell-permeable pyrazolidinedione compound that is shown to target RhoGEF DH-PH domain junction with high affinity (K
d = 65 nM) and effetively prevent RhoGEFs LARG, p115, and PDZ from interacting with RhoA, while displaying little potency against DBL-RhoA, LBC-RhoA, intersectin-Cdc42, or TrioN-Cdc42 interaction. Shown to completely prevent serum-induced activation of cellular RhoA, but not Cdc42 or Rac1, in NIH-3T3 cultures (10 µM) and RhoA downstream signaling events. Greatly synergizes with Rho GEF-binding domain blocker Rhosin (Cat. No.
555460) in blocking RhoA-LARG interaction and in preventing cellular RhoA activation (both drug at 5 µM).
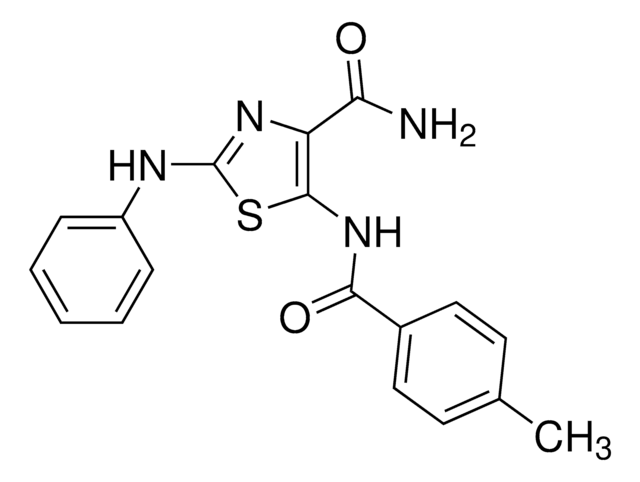
A cell-permeable pyrazolidinedione compound that prevents RhoGEFs LARG, p115, and PDZ from interacting with RhoA, while displaying little potency against DBL-RhoA, LBC-RhoA, intersectin-Cdc42, or TrioN-Cdc42 interaction. Structure and
in vitro binding studies using DH-PH domains-containing human LARG catalytic fragment (aa 765-1138) constructs reveals RhoGEF DH-PH domain junction as the high affinity Y16 targeting site (K
d = 65, 472, 2121, and >5 x 10
5 nM using wt, K979A, E982A, and N983A LARG, respectively). Shown to prevent serum-induced activation of cellular RhoA, but not Cdc42 or Rac1, in serum-starved NIH-3T3 cultures (215%, 109%, and 87% of basal RhoA-GTP level with 0, 10, or 30 µM Y16, respectively) and effectively inhibit RhoA downstream signaling events, including FAK and MLC phosphorylations and stress fiber formation without apparent cytotoxicity (50 µM up to 24 h) or affecting Cdc42-mediated filapodia and Rac1-mediated lamellipodia formation. Greatly synergizes with Rho GEF-binding domain blocker Rhosin (Cat. No.
555460) in blocking RhoA-LARG binding (8%, 27%, and 72% inhibition, respectively, with 5 µM G04 alone, 5 µM Y16 alone, or in combination) and in inhibiting cellular RhoA activation (80% inhibition of serum-stimulated RhoA-GTP level by 30 µM Y16 alone or by a combination of 5 µM each of G04 and Y16 in serum-starved NIH-3T3 cells).