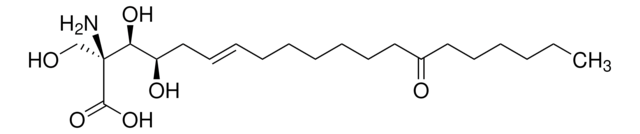
344850-M
Fumonisin B1, Fusarium moniliforme
A cell-permeable mycotoxin that inhibits sphingolipid biosynthesis in rat kidney and in liver microsomes by inhibition of sphingosine N-acyltransferase (ceramide synthase; IC₅₀ = 100 nM).
别名:
Fumonisin B1, Fusarium moniliforme, FB₁
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
品質等級
化驗
≥95% (TLC)
形狀
powder
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
顏色
white to beige
溶解度
methanol: 5 mg/mL
儲存溫度
2-8°C
InChI
1S/C34H59NO15/c1-5-6-9-20(3)32(50-31(44)17-23(34(47)48)15-29(41)42)27(49-30(43)16-22(33(45)46)14-28(39)40)13-19(2)12-24(36)10-7-8-11-25(37)18-26(38)21(4)35/h19-27,32,36-38H,5-18,35H2,1-4H3,(H,39,40)(H,41,42)(H,45,46)(H,47,48)/t19-,20+,21+,22+,23+,24+,25-,26+,27-,32-/m1/s1
InChI 密鑰
UVBUBMSSQKOIBE-ZWKVXHQASA-N
一般說明
A cell-permeable mycotoxin that inhibits sphingolipid biosynthesis in rat kidney and in liver microsomes by inhibition of sphingosine N-acyltransferase (ceramide synthase; IC50 = 100 nM). Preferentially inhibits sphingomyelin biosynthesis in neuronal cells. Exhibits carcinogenic properties.
Fumonisin B1 is a neurotoxin and a phytotoxin. A cell-permeable mycotoxin that inhibits sphingolipid biosynthesis in rat kidney and in liver microsomes by inhibition of sphingosine N-acyltransferase (ceramide synthase) (IC50 = 100 nM). Cellular effects also appear to be induced by micromolar levels of FB1. Because it inhibits ceramide synthase activity, it elevates cellular levels of sphingoid bases, including sphinganine, resulting in overall inhibition of sphingolipid biosynthesis. Preferentially inhibits sphingomyelin biosynthesis in neuronal cells. Has carcinogenic properties.
生化/生理作用
Primary Target
ceramide synthase
ceramide synthase
Target IC50: 100 nM against sphingosine N-acyltransferase (ceramide synthase)
警告
Toxicity: Carcinogenic / Teratogenic (D)
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 1 month at -20°C.
其他說明
Wu, W.I., et al. 1995. J. Biol. Chem.270, 13171.
Riley, R.T., et al. 1994. J. Nutr. 124, 594.
Wolf, G. 1994. Nutr. Rev. 52, 246.
Merrill, A.H. Jr., et al. 1993. Adv. Lipid Res.26, 215.
Merrill, Jr., A.H., et al. 1993. J. Biol. Chem.268, 27299.
Voss, K.A., et al. 1993. Nat. Toxins1, 222.
Wang, E., et al. 1991. J. Biol. Chem.266, 14486.
Harrison, L.R., et al. 1990. J. Vet. Diag. Invest. 2, 217.
Riley, R.T., et al. 1994. J. Nutr. 124, 594.
Wolf, G. 1994. Nutr. Rev. 52, 246.
Merrill, A.H. Jr., et al. 1993. Adv. Lipid Res.26, 215.
Merrill, Jr., A.H., et al. 1993. J. Biol. Chem.268, 27299.
Voss, K.A., et al. 1993. Nat. Toxins1, 222.
Wang, E., et al. 1991. J. Biol. Chem.266, 14486.
Harrison, L.R., et al. 1990. J. Vet. Diag. Invest. 2, 217.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Carc. 2 - Repr. 2 - STOT RE 2
標靶器官
Kidney,Liver
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门