推荐产品
等級
certified reference material
品質等級
形狀
liquid
特點
Snap-N-Spike®/Snap-N-Shoot®
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
濃度
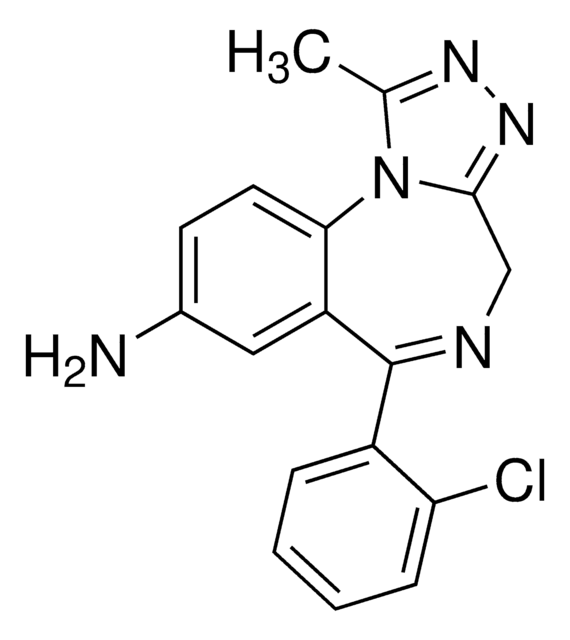
1.0 mg/mL in methanol
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
clinical testing
格式
single component solution
儲存溫度
−20°C
SMILES 字串
CN1C(=O)CC(=O)N(c2ccccc2)c3cc(Cl)ccc13
InChI
1S/C16H13ClN2O2/c1-18-13-8-7-11(17)9-14(13)19(16(21)10-15(18)20)12-5-3-2-4-6-12/h2-9H,10H2,1H3
InChI 密鑰
CXOXHMZGEKVPMT-UHFFFAOYSA-N
基因資訊
human ... GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562) , GABRD(2563) , GABRE(2564) , GABRG1(2565) , GABRG2(2566) , GABRG3(2567) , GABRP(2568) , GABRQ(55879)
一般說明
法律資訊
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
49.5 °F - closed cup
閃點(°C)
9.7 °C - closed cup
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门