922153
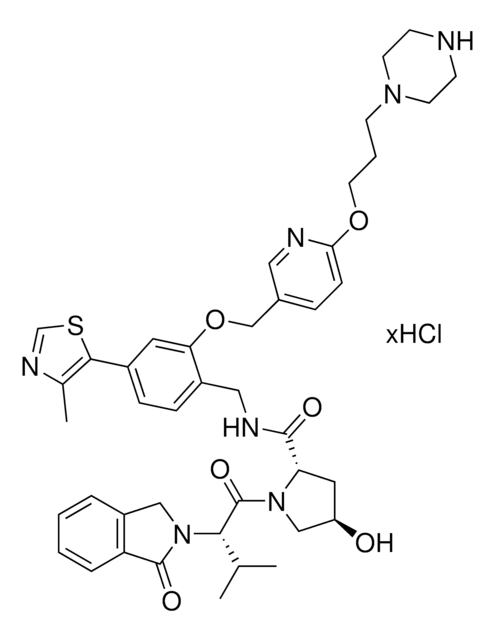
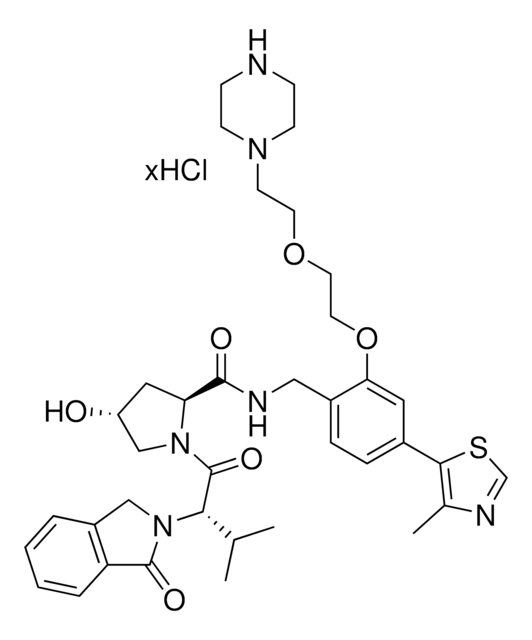
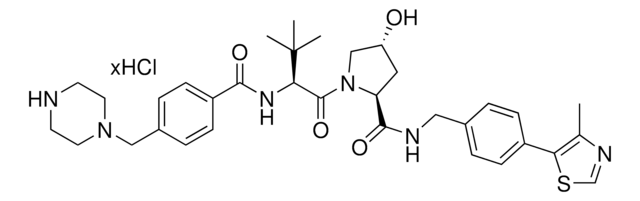
(S,R,S)-VL285 Phenol-C3-piperazine hydrochloride
别名:
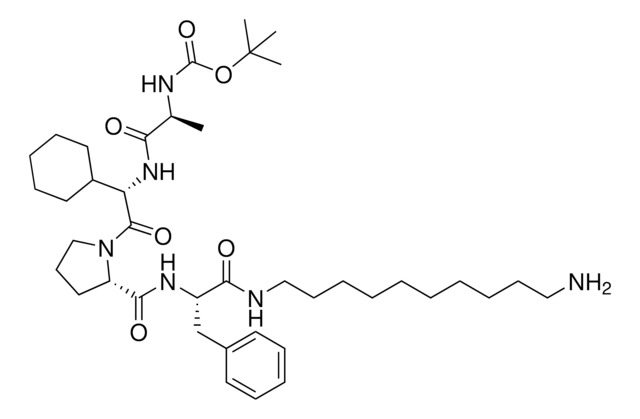
(2S,4R)-4-Hydroxy-1-((S)-3-methyl-2-(1-oxoisoindolin-2-yl)butanoyl)-N-(4-(4-methylthiazol-5-yl)-2-((6-(3-(piperazin-1-yl)propoxy)pyridin-3-yl)methoxy)benzyl)pyrrolidine-2-carboxamide, Crosslinker−E3 Ligase ligand conjugate, VHL protein degrader building block for PROTAC® research
选择尺寸
About This Item
推荐产品
ligand
VL285 phenol
质量水平
表单
solid
反应适用性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
官能团
amine
储存温度
2-8°C
SMILES字符串
O=C([C@@H]1C[C@@H](O)CN1C([C@H](C(C)C)N2CC(C=CC=C3)=C3C2=O)=O)NCC4=CC=C(C5=C(C)N=CS5)C=C4OCC6=CC=C(OCCCN7CCNCC7)C=N6.Cl
InChI
1S/C42H51N7O6S.ClH/c1-27(2)38(49-23-31-7-4-5-8-35(31)41(49)52)42(53)48-24-33(50)20-36(48)40(51)45-21-30-10-9-29(39-28(3)46-26-56-39)19-37(30)55-25-32-11-12-34(22-44-32)54-18-6-15-47-16-13-43-14-17-47;/h4-5,7-12,19,22,26-27,33,36,38,43,50H,6,13-18,20-21,23
InChI key
GMECTDIJBGAEOP-VLHWNADMSA-N
1 of 4
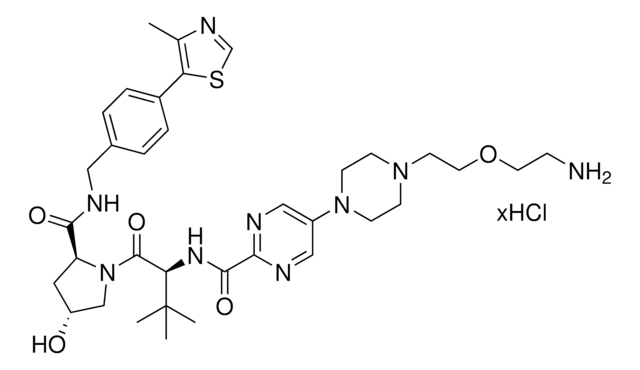
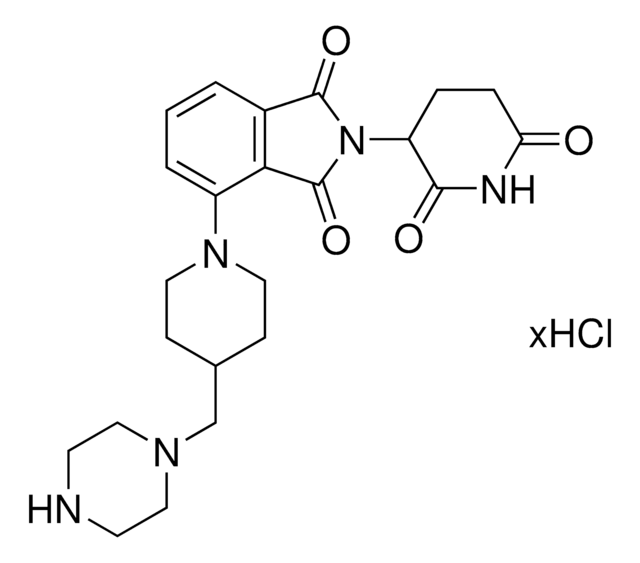
此商品 | 920843 | 920878 | 920894 |
|---|---|---|---|
| ligand VL285 phenol | ligand VL285 phenol | ligand VL285 phenol | ligand VL285 phenol |
| form solid | form solid | form solid | form solid |
| reaction suitability reactivity: carboxyl reactive | reaction suitability - | reaction suitability reactivity: carboxyl reactive, reagent type: ligand-linker conjugate | reaction suitability reactivity: carboxyl reactive, reagent type: ligand-linker conjugate |
| functional group amine | functional group - | functional group amine | functional group amine |
| Quality Level 100 | Quality Level 100 | Quality Level 100 | Quality Level 100 |
应用
其他说明
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins
Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase
法律信息
相关产品
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
商品
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持

![3-(([1,1′-Biphenyl]-4-ylmethyl)thio)-6-methyl-1,2,4,5-tetrazine >98%](/deepweb/assets/sigmaaldrich/product/structures/641/985/7d39c434-c9ea-490e-974f-7653ca3b9e8c/640/7d39c434-c9ea-490e-974f-7653ca3b9e8c.png)