907391
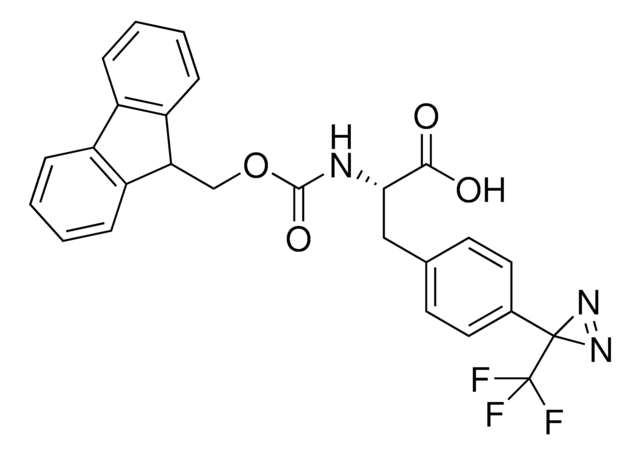
Fmoc-L-photo-leucine
≥98%
别名:
(S)-2-(((9H-Fluoren-9-yl)methoxy)carbonylamino)-3-(3-methyl-3H-diazirin-3-yl)propanoic acid, (S)-2-(Fmoc-amino)-3-(3H-diazirin-3-yl)butanoic acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
选择尺寸
About This Item
推荐产品
方案
≥98%
表单
powder
反应适用性
reaction type: Fmoc solid-phase peptide synthesis
应用
peptide synthesis
官能团
Fmoc
储存温度
2-8°C
SMILES字符串
N([C@@H](CC4(N=N4)C)C(=O)O)C(=O)OCC1c2c(cccc2)c3c1cccc3
InChI
1S/C20H19N3O4/c1-20(22-23-20)10-17(18(24)25)21-19(26)27-11-16-14-8-4-2-6-12(14)13-7-3-5-9-15(13)16/h2-9,16-17H,10-11H2,1H3,(H,21,26)(H,24,25)/t17-/m0/s1
InChI key
GDWMJFRPAHGSDU-KRWDZBQOSA-N
应用
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
其他说明
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers′
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
相关产品
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持