708739
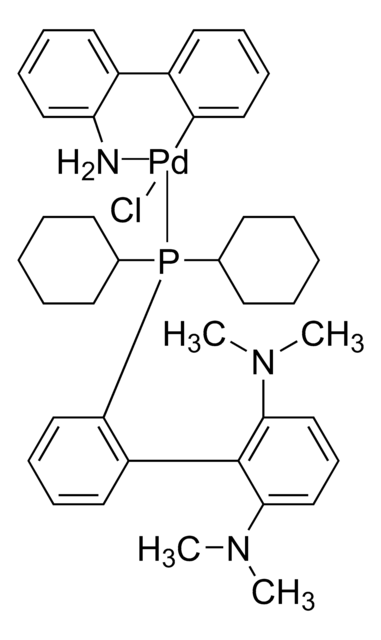
tBuXPhos Pd G1
别名:
t-BuXPhos palladium(II) phenethylamine chloride, tBuXPhos-Pd-G1, [2-(二叔丁基膦)-2′,4′,6′-三异丙基-1,1′-联苯基][2-(2-氨基乙基)苯基)]氯化钯(II), t-BuXPhos预催化剂, 氯[2-(二叔丁基膦)-2′,4′,6′-三异丙基-1,1′-联苯基][2-(2-氨基乙基)苯基)]钯(II), 氯[2-(二叔丁基膦基)-2',4',6'-三异丙基-1,1'-联苯基][2-(2-氨基乙基)苯基)]钯(II)
About This Item
推荐产品
形狀
solid
特點
generation 1
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
150-159 °C
官能基
phosphine
SMILES 字串
NCCc1ccccc1[Pd]Cl.CC(C)c2cc(C(C)C)c(c(c2)C(C)C)-c3ccccc3P(C(C)(C)C)C(C)(C)C
InChI
1S/C29H45P.C8H10N.ClH.Pd/c1-19(2)22-17-24(20(3)4)27(25(18-22)21(5)6)23-15-13-14-16-26(23)30(28(7,8)9)29(10,11)12;9-7-6-8-4-2-1-3-5-8;;/h13-21H,1-12H3;1-4H,6-7,9H2;1H;/q;;;+1/p-1
InChI 密鑰
LQRWNWRVOIDQOD-UHFFFAOYSA-M
法律資訊
訊號詞
Warning
危險分類
Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门







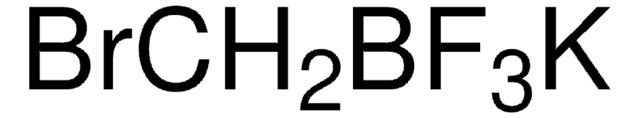


![氯(2-二环己基膦基-2′,6′-二甲氧基-1,1′-联苯基)[2-(2-氨基乙基苯基)]钯(II) - 甲基--叔丁基醚加合物](/deepweb/assets/sigmaaldrich/product/structures/421/182/4ca66fb3-8d93-499c-b88b-b8fe48ca97b8/640/4ca66fb3-8d93-499c-b88b-b8fe48ca97b8.png)


![氯-(2-二环己基膦-2′,6'-双异丙氧基-1,1′-联苯) [2-(2-氨乙基)苯基] 钯 (Ⅱ)-甲基--叔 -丁醚加合物 95%](/deepweb/assets/sigmaaldrich/product/structures/176/620/0ff76ec6-d361-4f7a-a777-45780811b3c0/640/0ff76ec6-d361-4f7a-a777-45780811b3c0.png)