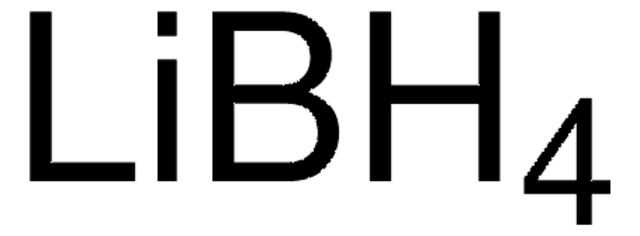推荐产品
形狀
liquid
反應適用性
reagent type: reductant
濃度
0.5 M in diethyl ether
密度
0.719 g/mL at 25 °C
SMILES 字串
[Li+].[H][B-]([H])([H])[H]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChI 密鑰
UUKMSDRCXNLYOO-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
反应物用于:
- 镓、铟、铼和锌三(巯基咪唑基)氢硼酸配合物的制备
- 机械化学复分解反应
- 非催化水解制氢
- 大型金单层保护簇的生长
- 阴离子取代反应
- 脱氢反应
Lithium borohydride is a commonly used reducing agent. It can also be used a reactant for:
- Preparation of gallium, indium, rhenium and zinc tris(mercaptoimidazolyl)hydroborato complexes.
- Mechano-chemical metathesis reactions.
- Noncatalytic hydrolysis for hydrogen generation.
- Growth of large gold monolayer protected-clusters.
- Anion substitution reactions.
- Dehydrogenation reactions.
其他說明
Material may form precipitate on standing, which does not affect its use.
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Central nervous system
安全危害
儲存類別代碼
4.3 - Hazardous materials which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 1
閃點(°F)
-40.0 °F
閃點(°C)
-40 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Mechano-chemical reactions in LiBH4+ VCln (n= 2 and 3) mixtures.
Llamas-Jansa I, et al.
J. Alloy Compounds, 509, S684-S687 (2011)
Controlled growth and catalytic activity of gold monolayer protected clusters in presence of borohydride salts.
Dasog M, et al.
Chemical Communications (Cambridge, England), 47(30), 8569-8571 (2011)
Effect of hydrogen back pressure on dehydrogenation behavior of LiBH4-based reactive hydride composites.
Shim J H, et al.
The Journal of Physical Chemistry Letters, 1(1), 59-63 (2009)
Lithium Borohydride.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2009)
Synthesis, crystal structure, and thermal properties of the first mixed-metal and anion-substituted rare earth borohydride LiCe (BH4) 3Cl.
Frommen c, et al.
The Journal of Physical Chemistry, 115(47), 23591-23602 (2011)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门