推荐产品
方案
97%
表单
powder
旋光性
[α]22/D +337°, c = 1 in chloroform
mp
90-96 °C
储存温度
−20°C
InChI
1S/C33H32NO2P/c1-23(25-11-5-3-6-12-25)34(24(2)26-13-7-4-8-14-26)37-35-29-17-9-15-27-19-21-33(31(27)29)22-20-28-16-10-18-30(36-37)32(28)33/h3-18,23-24H,19-22H2,1-2H3/t23-,24-,33?/m1/s1
InChI key
ZXLQLIDJAURBDD-HRPAVAKOSA-N
应用
(R)-SIPHOS-PE can be used as:
- A catalyst in the preparation of tricyclic alkaloid (+)-aphanorphine via asymmetric carboamination and Friedel−Crafts reaction.[1]
- A ligand in the synthesis of chiral ketones via hydroacylation of olefins using salicylaldehyde derivatives.[2]
- A ligand in the enantioselective synthesis of substituted pyrrolidine derivatives via Pd-catalyzed alkene aminoarylation reactions.[3]
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
储存分类代码
13 - Non Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
Recent developments in palladium-catalyzed alkene aminoarylation reactions for the synthesis of nitrogen heterocycles
Schultz DM and Wolfe JP
Synthesis, 44(03), 351-361 (2012)
Regio-and enantioselective intermolecular hydroacylation: Substrate-directed addition of salicylaldehydes to homoallylic sulfides
Coulter MM, et al.
Journal of the American Chemical Society, 132(46), 16330-16333 (2010)
Enantioconvergent synthesis of (+)-aphanorphine via asymmetric Pd-catalyzed alkene carboamination
Mai DN, et al.
Organic Letters, 13(11), 2932-2935 (2011)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门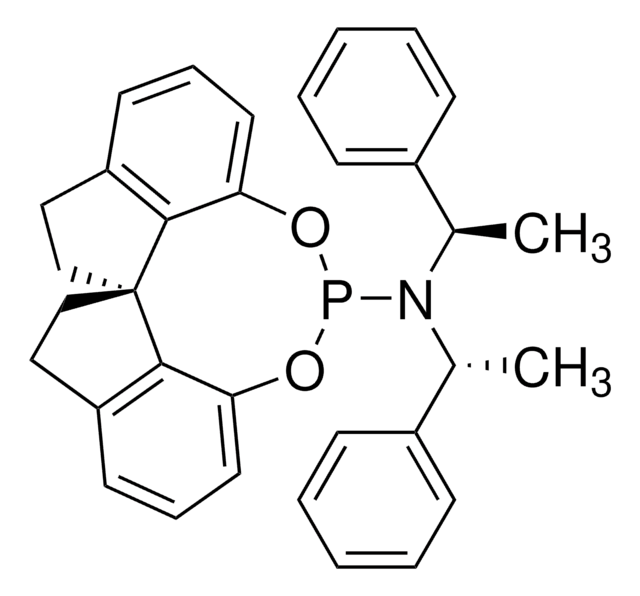


![(R)-(-)-1-[(S)-2-二苯基磷]二茂铁乙基二环己基磷 ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)




![(S)-(+)-1-[(R)-2-(二苯基膦)二茂铁]乙基二环己基膦 ≥97%](/deepweb/assets/sigmaaldrich/product/structures/259/060/e5169bde-8514-471f-aaaa-45e78b6055d2/640/e5169bde-8514-471f-aaaa-45e78b6055d2.png)
