所有图片(3)
About This Item
经验公式(希尔记法):
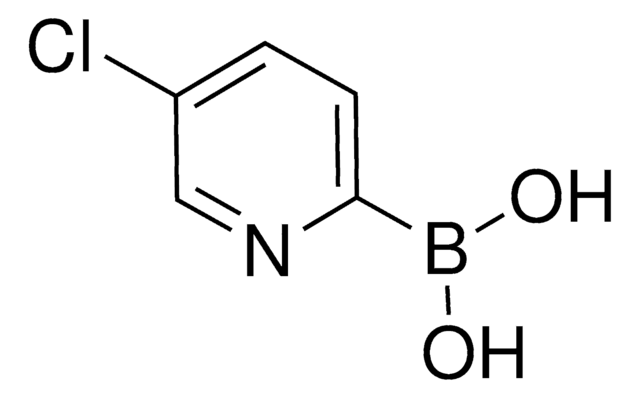
C5H5BClNO2
CAS号:
分子量:
157.36
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥95.0%
形狀
solid
mp
165 °C (lit.)
SMILES 字串
OB(O)c1ccc(Cl)nc1
InChI
1S/C5H5BClNO2/c7-5-2-1-4(3-8-5)6(9)10/h1-3,9-10H
InChI 密鑰
WPAPNCXMYWRTTL-UHFFFAOYSA-N
應用
6-Chloro-3-pyridinylboronic acid can be used:
- To prepare biologically significant 3-arylcoumarins by reacting with 3-chlorocoumarin through Suzuki reaction.
- As a substrate in the synthesis of 11-(pyridinylphenyl)steroid with progesterone agonist/antagonist profile.
- As a substrate in the preparation of α- secondary and tertiary pyridines by the reaction of pyridotriazoles with boronic acids.
- As a substrate in the palladium-catalyzed α-arylation of saturated cyclic amines and N-methyl amines.
其他說明
含有不定量的酸酐
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Synthesis of 3-arylcoumarins via Suzuki-cross-coupling reactions of 3-chlorocoumarin
Matos MJ, et al.
Tetrahedron Letters, 52(11), 1225-1227 (2011)
11-(Pyridinylphenyl) steroids-A new class of mixed-profile progesterone agonists/antagonists
Rewinkel J, et al.
Bioorganic & Medicinal Chemistry, 16(6), 2753-2763 (2008)
Metal-Free Denitrogenative C-C Couplings of Pyridotriazoles with Boronic Acids To Afford α-Secondary and α-Tertiary Pyridines
Dong C, et al.
Organic Letters, 21(11), 4148-4152 (2019)
α-Arylation of Saturated Azacycles and N-Methylamines via Palladium (II)-catalyzed C (sp3)-H Coupling
Spangler JE, et al.
Journal of the American Chemical Society, 137(37), 11876-11879 (2015)
商品
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
相关内容
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门