推荐产品
化驗
97%
形狀
solid
mp
163-168 °C (lit.)
SMILES 字串
Cc1n[nH]c(C)c1B2OC(C)(C)C(C)(C)O2
InChI
1S/C11H19BN2O2/c1-7-9(8(2)14-13-7)12-15-10(3,4)11(5,6)16-12/h1-6H3,(H,13,14)
InChI 密鑰
GNUDAJTUCJEBEI-UHFFFAOYSA-N
應用
3,5-Dimethylpyrazole-4-boronic acid pinacol ester can be used:
- To synthesize 9H-pyrimido[4,5-b]indole and aryl-benzimidazole based BET bromodomain and extra terminal (BET) protein inhibitors.
- To prepare naphthalimide based photo-exchangeable photochromic fluorescent molecules.
- As a reactant to develop DNA-encoded chemical libraries by palladium-catalyzed Suzuki coupling reaction with DNA-linked aryl halides.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Investigation of Photochromic Fluorescence Features and Synthesis of Diarylethene Type Naphthalimide Compounds
Orhan E and Narin M
Journal of the Turkish Chemical Society Section A: Chemistry, 7(1), 97-106 (2020)
Structure-Based Discovery of 4-(6-Methoxy-2-methyl-4-(quinolin-4-yl)-9 H-pyrimido [4, 5-b] indol-7-yl)-3, 5-dimethylisoxazole (CD161) as a Potent and Orally Bioavailable BET Bromodomain Inhibitor
Zhao Y, et al.
Journal of medicinal chemistry, 60(9), 3887-3901 (2017)
Development of DNA-compatible suzuki-miyaura reaction in aqueous media
Li J and Huang H
Bioconjugate Chemistry, 29(11), 3841-3846 (2018)
Structure-guided discovery of a novel, potent, and orally bioavailable 3, 5-dimethylisoxazole aryl-benzimidazole BET bromodomain inhibitor
Sperandio D, et al.
Bioorganic & Medicinal Chemistry, 27(3), 457-469 (2019)
商品
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
相关内容
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门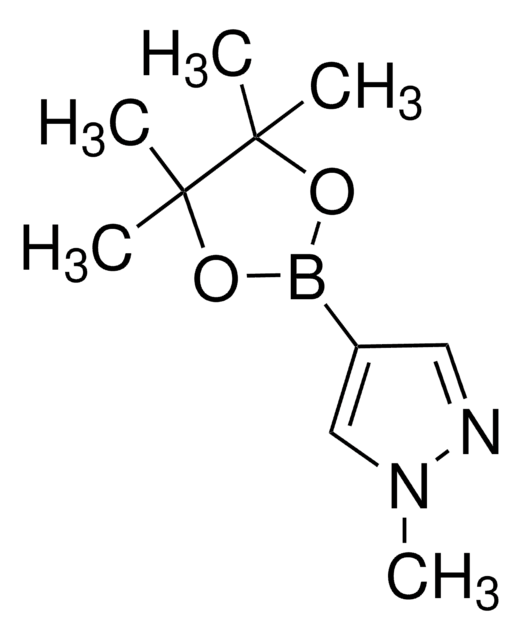

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)