所有图片(2)
About This Item
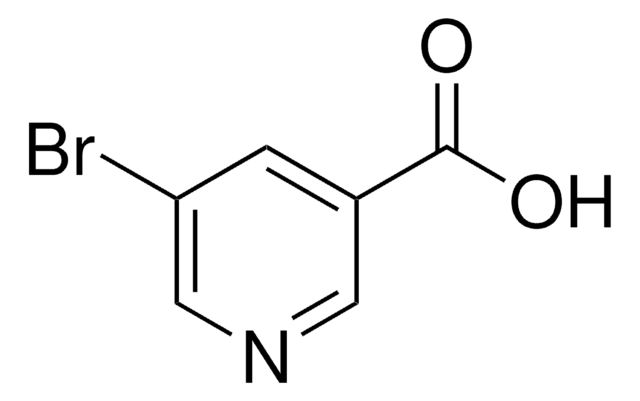
线性分子式:
IC6H3(CH3)NH2
CAS号:
分子量:
233.05
EC 号:
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
97%
表单
solid
mp
37-41 °C (lit.)
官能团
iodo
SMILES字符串
Cc1ccc(N)cc1I
InChI
1S/C7H8IN/c1-5-2-3-6(9)4-7(5)8/h2-4H,9H2,1H3
InChI key
RRUDMHNAMZFNEK-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
应用
3-Iodo-4-methylaniline can undergo reaction with paraformaldehyde to give the corresponding dihalo-substituted analogs of Troger′s base.[1] It can be also be used in the synthesis of BIRB 796 (1-(5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy)naphthalen-1-yl]urea), a promising agent for the treatment of inflammatory diseases.[2]
警示用语:
Danger
危险声明
危险分类
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Sens. 1
储存分类代码
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
"Synthesis of Dihalo-Substituted Analogues of Troger?s Base from ortho-and meta-Substituted Anilines"
Hansson A, et al.
European Journal of Organic Chemistry, 2003(16), 3179-3188 (2003)
"Synthesis of deuterium, tritium, and carbon-14 labeled BIRB 796, a p38 MAP kinase inhibitor"
Latli B
Journal of Labelled Compounds & Radiopharmaceuticals, 47(12), 847-856 (2004)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)