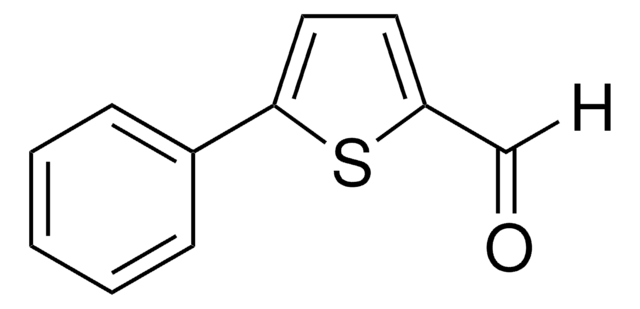
所有图片(1)
About This Item
经验公式(希尔记法):
C9H6OS2
CAS号:
分子量:
194.27
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
98%
mp
55-58 °C (lit.)
官能团
aldehyde
SMILES字符串
[H]C(=O)c1ccc(s1)-c2cccs2
InChI
1S/C9H6OS2/c10-6-7-3-4-9(12-7)8-2-1-5-11-8/h1-6H
InChI key
FYBWRAXKYXTOQC-UHFFFAOYSA-N
应用
2,2′-Bithiophene-5-carboxaldehyde may be used in the synthesis of the following:
- boron dipyrromethene(BODIPY)oligothiophenes via a multi-step reaction process[1]
- (2,2′-bithiophene-5-carbaldehyde)-4-nitrophenylhydrazone(BT-NPH) via reaction with 4-nitrophenylhydrazine[2]
- bithiophene fulleropyrrolidine obtained via refluxing with sarcosine and fullerene[3]
- azomethine phthalic diimides by heating with N,N-bis(4-amino-2,3,5,6-tetramethylphenyl)naphthalene-1,4,5,8-dicarboximide (DANDI)[4]
储存分类代码
13 - Non Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
其他客户在看
"The effect of thiophene substituents of fulleropyrrolidine acceptors on the performance of inverted organic solar cells"
Kaunisto.MK, et al.
Synthetic Metals, 195, 193-200 (2014)
"Optical nonlinearities and molecular conformations in thiophene-based hydrazone crystals"
Kwon P-O, et al.
The Journal of Physical Chemistry C, 113(34), 15405-15411 (2009)
"New low band gap compounds comprised of naphthalene diimide and imine units"
Schab-Balcerzak E, et al.
Synthetic Metals, 162(05), 543- 553 (2012)
"Enhanced Functionality for Donor?Acceptor Oligothiophenes by means of Inclusion of Bodipy: Synthesis, Electrochemistry, Photophysics, and Model Chemistry"
Collado D, et al.
Chemistry?A European Journal , 17(02), 498-507 (2011)
Sara S M Fernandes et al.
ACS omega, 3(10), 12893-12904 (2018-11-10)
A series of push-pull heterocyclic N,N-diphenylhydrazones were prepared to study the effect of structural modifications (different π-spacers and electron-withdrawing groups) on the optical (linear and nonlinear) and electronic properties of the molecules. The photovoltaic response of dye-sensitized solar cells assembled
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![苯并[b]噻吩-2-甲醛 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)

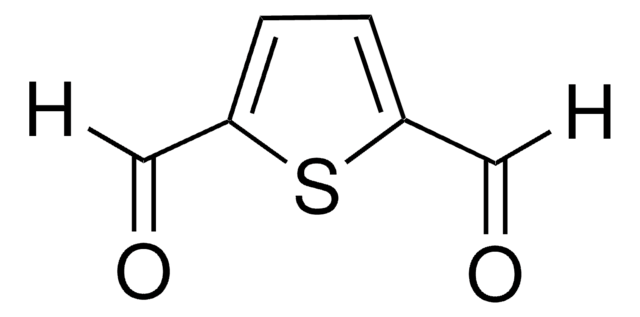
![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)