所有图片(1)
About This Item
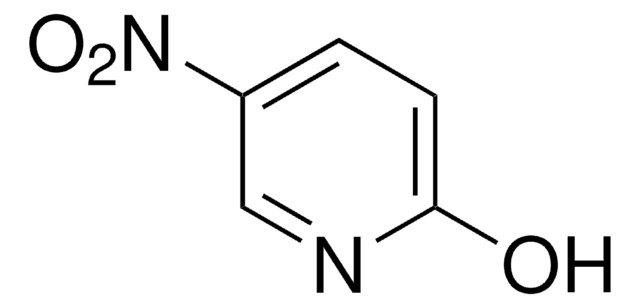
线性分子式:
(C6H5CH2COS)2
CAS号:
分子量:
302.41
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
96%
形狀
solid
mp
59-63 °C (lit.)
SMILES 字串
O=C(Cc1ccccc1)SSC(=O)Cc2ccccc2
InChI
1S/C16H14O2S2/c17-15(11-13-7-3-1-4-8-13)19-20-16(18)12-14-9-5-2-6-10-14/h1-10H,11-12H2
InChI 密鑰
IXGZXXBJSZISOO-UHFFFAOYSA-N
一般說明
苯乙酰二硫化物在磷酸盐制备过程中用作硫化试剂。苯乙硫醇磺酸盐与硫代乙酸在三乙胺的作用下反应可合成苯基乙酰二硫化物。
應用
苯乙酰二硫化物(PADS)可作为硫转移剂用于硫代磷酸寡核苷酸。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Caged O-phosphorothioyl amino acids as building blocks for Fmoc-based solid phase peptide synthesis.
Andreas Aemissegger et al.
Tetrahedron, 63(27), 6185-6190 (2007-07-02)
The synthesis of 1-(2-nitrophenylethyl) caged O-phosphorothioylserine, -threonine and -tyrosine derivatives is reported. These amino acid building blocks can be directly incorporated into peptides by Fmoc-based solid phase synthesis as their pentafluorophenyl esters or as symmetric anhydrides. Upon irradiation with UV
A study on the use of phenylacetyl disulfide in the solid-phase synthesis of oligodeoxynucleoside phosphorothioates.
Roelen HCPF, et al.
J. R. Neth. Chem. Soc., 110(7-8), 325-331 (1991)
"Organic disulfides and related substances. XXIII. Unsymmetrical carbonyl disulfides and cognate compounds"
Fiel L and Buckman DJ
The Journal of Organic Chemistry, 32(11), 3467-3470 (1967)
Honglu Zhang et al.
Journal of the American Chemical Society, 128(51), 16464-16465 (2006-12-21)
The activation of phosphatidylinositol 3-kinase (PI 3-K) and subsequent production of PtdIns(3,4,5)P3 launches a signal transduction cascade that impinges on a plethora of downstream effects on cell physiology. Control of PI 3-K and PtdIns(3,4,5)P3 levels is an important therapeutic target
Use of phenylacetyl disulfide (PADS) in the synthesis of oligodeoxyribonucleotide phosphorothioates.
Cheruvallath ZS, et al.
Nucleosides, nucleotides & nucleic acids, 18(3), 485-492 (1999)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门