所有图片(1)
About This Item
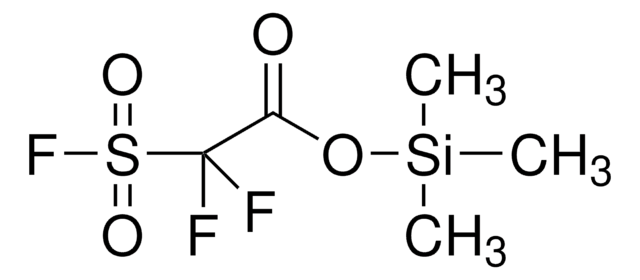
线性分子式:
FSO2CF2CO2H
CAS号:
分子量:
178.09
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
反應適用性
reaction type: click chemistry
折射率
n20/D 1.36 (lit.)
bp
153 °C (lit.)
密度
1.723 g/mL at 25 °C (lit.)
SMILES 字串
OC(=O)C(F)(F)S(F)(=O)=O
InChI
1S/C2HF3O4S/c3-2(4,1(6)7)10(5,8)9/h(H,6,7)
InChI 密鑰
VYDQUABHDFWIIX-UHFFFAOYSA-N
一般說明
2,2-二氟-2-(氟磺酰基)乙酸试剂可用作酚羟基二氟甲基化的二氟卡宾来源。
應用
2,2-二氟-2-(氟磺酰基)乙酸可被用于以下过程:
- 通过与相应的2-氯吡啶反应制备1-二氟甲基-2-氧代-1,2-二氢吡啶类似物。
- 制备作为新型高效二氟碳试剂的氟磺酰二氟乙酸硅酯用于烯烃的环丙烷化反应。
- 末端烯烃和炔烃与碘二氟甲烷磺酰胺的区域和立体选择性自由基氟烷基化反应。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 2 Oral - Skin Corr. 1A
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Trimethylsilyl fluorosulfonyldifluoroacetate (TFDA): a new, highly efficient difluorocarbene reagent
Dolbier, William R.; et al.
Journal of Fluorine Chemistry, 125, 459-469 (2004)
Free radical fluoroalkylation of terminal alkenes and alkynes with iododifluoromethanesulfonamides
Zhu, J. M.; et al.
Science China: Chemistry, 54, 95-102 (2011)
Free radical fluoroalkylation of terminal alkenes and alkynes with iododifluoromethanesulfonamides.
Zhu JM, et al.
Science China: Chemistry, 54(1), 95-102 (2011)
Preparation and use of a new difluorocarbene reagent
Dolbier, W. R., Jr.; et al.
Organic Syntheses, 80, 172-176 (2003)
Makoto Ando et al.
Organic letters, 8(17), 3805-3808 (2006-08-11)
[reaction: see text] A novel one-pot synthesis of N-difluoromethyl-2-pyridones is described. N-(Pyridin-2-yl)acetamide derivatives were excellent precursors for the preparation of N-difluoromethyl-2-pyridone derivatives. Difluoromethylation of 2-acetaminopyridine derivatives was achieved with sodium chlorodifluoroacetate as a difluorocarbene source in the presence of a
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门



![4-(乙酰氨基)苯基 ] 咪唑二磺酰二氟化物 ≥98%](/deepweb/assets/sigmaaldrich/product/structures/101/806/3f40354f-e903-4ea0-9654-10872377816c/640/3f40354f-e903-4ea0-9654-10872377816c.png)