530263
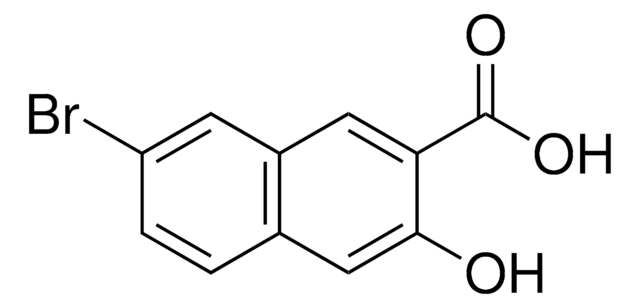
6-氰基-2-萘酚
97%
别名:
2-Cyano-6-hydroxynaphthalene, 2-Cyano-6-naphthol, 2-Hydroxy-6-naphthonitrile, 6-Cyano-2-hydroxynaphthalene, 6-Hydroxy-2-naphthalenecarbonitrile, 6-Hydroxy-2-naphthonitrile
登录查看公司和协议定价
所有图片(1)
选择尺寸
变更视图
25 G
$502.00
About This Item
线性分子式:
NCC10H6OH
CAS号:
分子量:
169.18
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
质量水平
方案
97%
mp
165.5-170.5 °C (lit.)
官能团
nitrile
SMILES字符串
Oc1ccc2cc(ccc2c1)C#N
InChI
1S/C11H7NO/c12-7-8-1-2-10-6-11(13)4-3-9(10)5-8/h1-6,13H
InChI key
WKTNIBWKHNIPQR-UHFFFAOYSA-N
一般描述
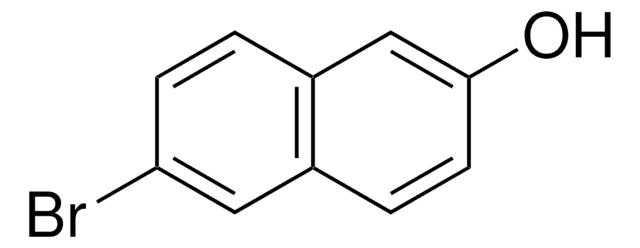
6-Cyano-2-naphthol (6CN2) is an aromatic alcohol that can be synthesized from 6-bromo-2-naphthol.[1] It is a superphotoacid with the ground state pKa* value of 8.4 and excited state pKavalue of 0.2, respectively. 6CN2 protonates PANI-ES (polyaniline emeraldine salt) to form PANI-EB (emeraldine base), which shows enhanced conductivity.[1] The proton-transfer kinetics and photophysical behavior of 6CN2 have been investigated.[2]
应用
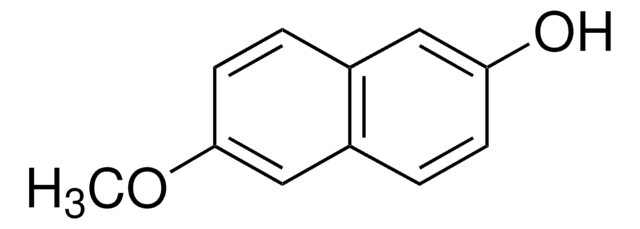
6-Cyano-2-naphthol (6-Hydroxy-2-naphthonitrile, 2-cyano-6-naphthol) may be used in the preparation of:
- 5-bromo-6-hydroxy-2-naphthonitrile[3]
- 5,7-dibromo-6-hydroxy-2-naphthonitrile[3]
- 5-chloro-6-hydroxy-2-naphthonitrile[3]
- 6-(2-imidazolyl)-2-naphthol[4]
- dodecaethylene glycol di-6-cyano-2-naphthyl ether[5]
- 6-cyano-2-naphthyl trifluoremethanesufonate[6]
- 2-(6-cyano-naphthyl)2,3,4-tri-O-acetyl-β-D-xylopyranoside[7]
- 1,5-bis(7-amidino-2-naphthalenoxy)-3-oxapentane dihydrochloride[8]
Reactant for:
- Palladium-catalyzed reduction
- Nickel-catalyzed cross-coupling reactions
- Palladium-catalyzed Heck reactions
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Doping of Polyaniline with 6-Cyano-2-naphthol.
Das D, et al.
The Journal of Physical Chemistry B, 118(45), 12993-13001 (2014)
Anna Siegbahn et al.
Organic & biomolecular chemistry, 13(11), 3351-3362 (2015-02-07)
Proteoglycans (PGs) are macromolecules that consist of long linear polysaccharides, glycosaminoglycan (GAG) chains, covalently attached to a core protein by the carbohydrate xylose. The biosynthesis of GAG chains is initiated by xylosylation of the core protein followed by galactosylation by
Ultrafast excited-state proton transfer from cyano-substituted 2-naphthols.
The Journal of Physical Chemistry A, 101(25), 4602-4605 (1997)
Maryam Rahimian et al.
Biochemistry, 48(7), 1573-1583 (2009-01-29)
Most A/T specific heterocyclic diamidine derivatives need at least four A/T base pairs for tight binding to the DNA minor groove. Addition of a GC base pair to A/T sequences typically causes a large decrease in binding constant. The ability
T Nakayama et al.
Chemical & pharmaceutical bulletin, 41(1), 117-125 (1993-01-01)
By developing 6-amidino-2-naphthyl 4-guanidinobenzoate (I, FUT-175) as a basic structure, its various derivatives were synthesized and their inhibitory activities on trypsin, plasmin, kallikrein, thrombin, C1r and C1s as well as on complement-mediated hemolysis were examined. The protective effect of these
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持