所有图片(1)
About This Item
经验公式(希尔记法):
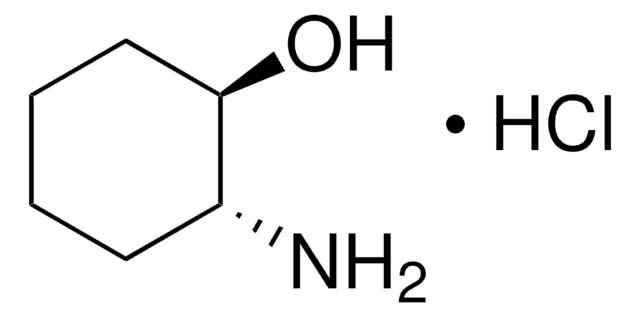
C5H11NO · HCl
CAS号:
分子量:
137.61
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
97%
mp
191-196 °C (lit.)
官能团
hydroxyl
SMILES字符串
Cl.N[C@H]1CCC[C@@H]1O
InChI
1S/C5H11NO.ClH/c6-4-2-1-3-5(4)7;/h4-5,7H,1-3,6H2;1H/t4-,5-;/m0./s1
InChI key
ZFSXKSSWYSZPGQ-FHAQVOQBSA-N
一般描述
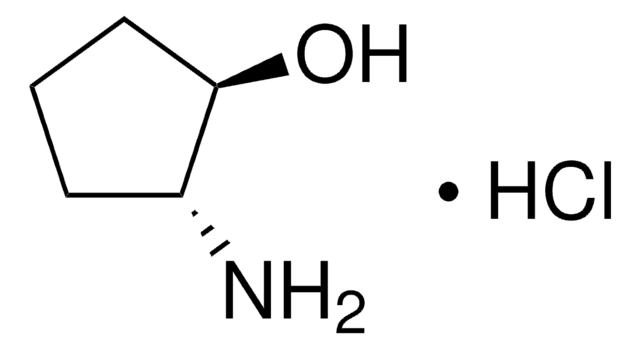
trans-2-Aminocyclopentanol hydrochloride is an aminocyclanol.[1] Its d,l-cis- and d,l-trans- forms have been synthesized.[2] The trans-form of the product can be produced in large (multigram) scale via carbamate addition protocol.[3] Cholinesterase inhibitory potential of cis-form of 2-aminocyclopentanol hydrochloride is higher (twofold) that of its trans-form.[1]
储存分类代码
13 - Non Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
James A Birrell et al.
Organic letters, 15(12), 2895-2897 (2013-06-08)
A highly enantioselective addition of phenyl carbamate to meso-epoxides has been developed to efficiently generate protected trans-1,2-amino alcohols. This transformation is promoted by an oligomeric (salen)Co-OTf catalyst and has been used to prepare two useful 2-aminocycloalkanol hydrochlorides in enantiopure form
Stereochemistry of Aminocyclanols. Synthesis of cis Epimers via Oxazolines. The 2-Aminocyclopentanols*.
McCasland GE and Smith DA.
Journal of the American Chemical Society, 72(5), 2190-2195 (1950)
Preparation of antidotes for anticholinesterase poisoning. IV. Synthesis and protective effectiveness of 2'-(cis-and trans-2'-hydroxycyclohexyl) aminoethyl 1-phenylcyclopentanecarboxylate hydrochlorides.
Bannard RAB and Parkkari JH.
Canadian Journal of Chemistry, 48(9), 1377-1382 (1970)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持