所有图片(1)
About This Item
经验公式(希尔记法):
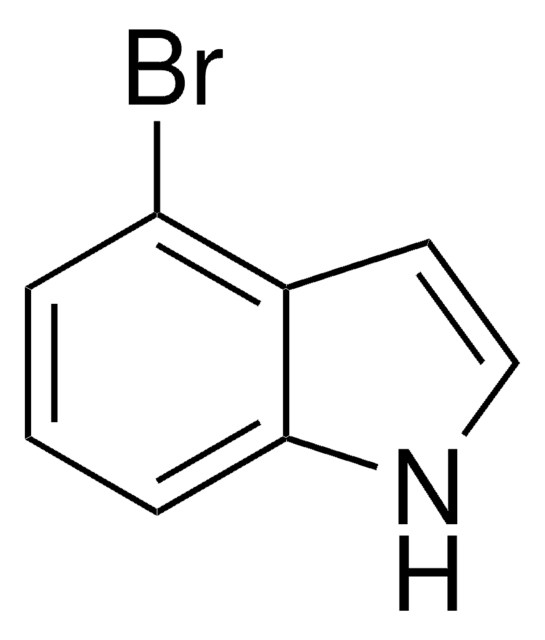
C8H6BrN
CAS号:
分子量:
196.04
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
96%
mp
92-96 °C (lit.)
SMILES 字串
Brc1ccc2cc[nH]c2c1
InChI
1S/C8H6BrN/c9-7-2-1-6-3-4-10-8(6)5-7/h1-5,10H
InChI 密鑰
MAWGHOPSCKCTPA-UHFFFAOYSA-N
一般說明
6-溴吲哚是一种吲哚衍生物。它可与2-(4-氟苯基)乙基哌嗪进行钯催化反应得到羰基化产物。
應用
6-取代吲哚化学中必需的起始剂。
6-溴吲哚可被用于合成:
- 6-烷基硫代吲哚
- 3-乙酰氧基-6-溴吲哚
- 6,6′-二溴靛蓝(泰尔紫)
- 6-酰基吲哚
- 6-溴吲哚-1-羧酸叔丁酯
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Synthesis of N-protected Nortopsentins B and D.
Moody CJ and Roffey JRA.
ARKIVOC (Gainesville, FL, United States), 1, 393-401 (2000)
Efficient synthesis of 5-and 6-tributylstannylindoles and their reactivity with acid chlorides in the Stille coupling reaction.
Cherry K, et al.
Tetrahedron Letters, 48(33), 5751-5753 (2007)
James R Fuchs et al.
Journal of the American Chemical Society, 126(16), 5068-5069 (2004-04-22)
The first total synthesis of racemic perophoramidine is described. The key step features the highly stereoselective introduction of the vicinial quaternary centers via base-promoted carbon-carbon bond formation between a 3-alkylindole and a 3-bromo-3-alkylindolin-2-one. This transformation presumably proceeds through a conjugate
A facile synthesis of Tyrian purple based on a biosynthetic pathway.
Tanoue Y, et al.
Fisheries Science (Tokyo, Japan), 67(4), 726-729 (2001)
Palladium-catalyzed carbonylation of haloindoles: No need for protecting groups.
Kumar K, et al.
Organic Letters, 6(1), 7-10 (2004)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门