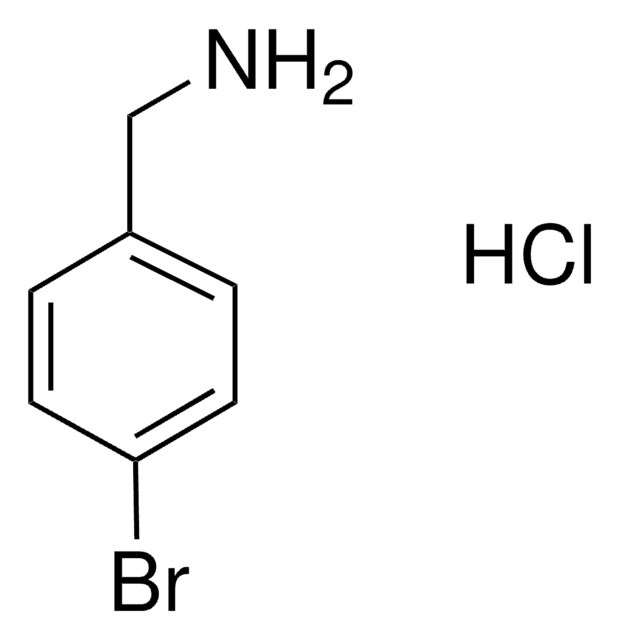
推荐产品
化驗
96%
bp
110-112 °C/30 mmHg (lit.)
mp
25 °C (lit.)
密度
1.473 g/mL at 25 °C (lit.)
SMILES 字串
NCc1ccc(Br)cc1
InChI
1S/C7H8BrN/c8-7-3-1-6(5-9)2-4-7/h1-4H,5,9H2
InChI 密鑰
XRNVSPDQTPVECU-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-溴苄胺(BBA)又名 p-溴苄胺,是一种芳基溴化物。据报道,在存在红铜的条件下,其可由BBA选择性地形成腈或亚胺。 BBA形成2,6,9-三氮杂双环[3.3.1]壬烷衍生物的formal[4 + 4]反应已有研究。
應用
4-溴苄胺(p-溴苄胺)可用于合成7-[(p-溴苄基)脲基]-7,8-二氢-α-双丁二烯。
它可用于合成以下4-联苯甲胺衍生物:
它可用于合成以下4-联苯甲胺衍生物:
- (4′-氟-4-联苯)甲胺
- (4′-甲氧基-4-联苯)甲胺
- (2′-甲氧基-4-联苯)甲胺
- (3′-氰基-4-联苯)甲胺
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
>230.0 °F - closed cup
閃點(°C)
> 110 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Nitrogenous bisabolene sesquiterpenes from marine invertebrates.
Gulavita NK, et al.
The Journal of Organic Chemistry, 51(26), 5136-5139 (1986)
Jing Zhuang et al.
Nanoscale, 11(31), 14553-14560 (2019-07-26)
An all-inorganic CsPbI2Br perovskite with excellent phase stability and thermal stability has been considered to be a promising candidate for photovoltaic application. However, low efficiency and high moisture sensitivity hinder its advancement. In this work, we exploit 4-bromobenzylamine hydriodate post-treatment
Silvia Galiano et al.
Bioorganic & medicinal chemistry, 15(11), 3896-3911 (2007-04-05)
We have designed and synthesized two novel series of MCH-R1 antagonists based on a substituted biphenylmethyl urea core. SAR was explored, suggesting that optimal binding with the receptor was achieved when the biphenylmethyl group and the linker were substituted on
Jiaqing Wang et al.
Chemical communications (Cambridge, England), 50(42), 5637-5640 (2014-04-16)
A novel, efficient, convenient and environmentally friendly approach for the synthesis of nitriles and imines from primary amines has been developed. Using commercially available red copper as the catalyst, ammonium bromide as the co-catalyst and molecular oxygen as the sole
Ho Yeon Nam et al.
Biopolymers, 106(1), 82-88 (2015-09-26)
We developed a new method for modifying the side chains of peptoids on a solid phase resin, employing the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction. Optimized conditions using Pd(PPh3 )4 and K2 CO3 in the presence of Buchwald's SPhos ligand provided a
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门