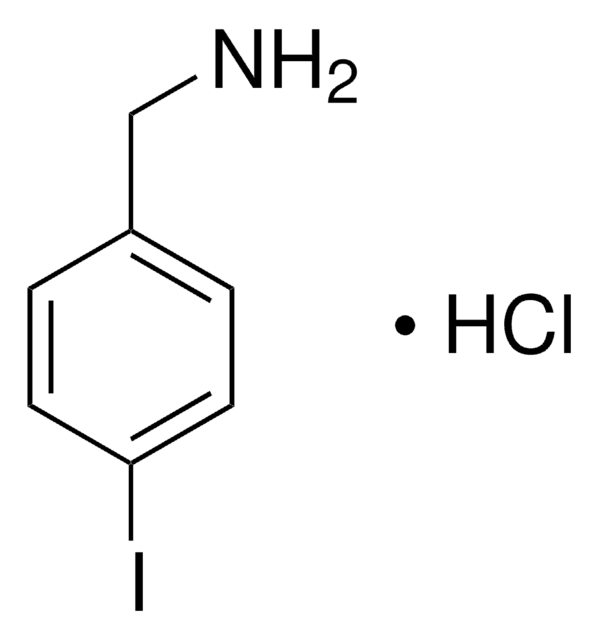
推荐产品
化驗
97%
mp
188-190 °C (lit.)
SMILES 字串
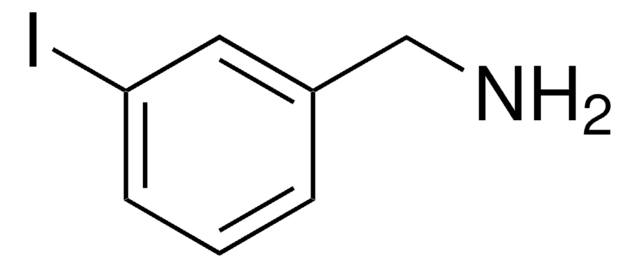
Cl.NCc1cccc(I)c1
InChI
1S/C7H8IN.ClH/c8-7-3-1-2-6(4-7)5-9;/h1-4H,5,9H2;1H
InChI 密鑰
PYFDZOCGFHIRST-UHFFFAOYSA-N
應用
3-Iodobenzylamine hydrochloride was used as the starting reagent in the synthesis of N6-(3-iodobenzyl)-2-substituted-adenosine derivatives. It was used in the synthesis of 3′-C-methyl adenosine N6-substituted and N6/C-2 disubstituted derivatives and novel 2′-C-methyl analogues.
訊號詞
Danger
危險分類
Eye Irrit. 2 - Repr. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
H O Kim et al.
Journal of medicinal chemistry, 37(21), 3614-3621 (1994-10-14)
Adenosine derivatives bearing an N6-(3-iodobenzyl) group, reported to enhance the affinity of adenosine-5'-uronamide analogues as agonists at A3 adenosine receptors (J. Med. Chem. 1994, 37, 636-646), were synthesized starting from methyl beta-D-ribofuranoside in 10 steps. Binding affinities at A1 and
Loredana Cappellacci et al.
Journal of medicinal chemistry, 48(5), 1550-1562 (2005-03-04)
A number of 3'-C-methyl analogues of selective adenosine receptor agonists such as CPA, CHA, CCPA, 2'-Me-CCPA, NECA, and IB-MECA was synthesized to further investigate the subdomain of the receptor that binds the ribose moiety of the ligands. Affinity data at
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门