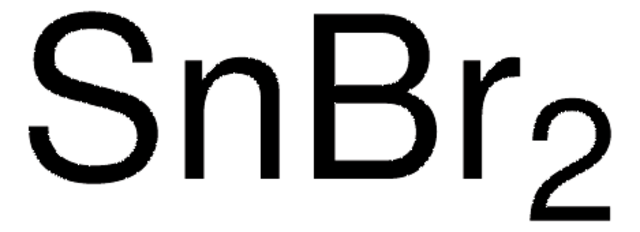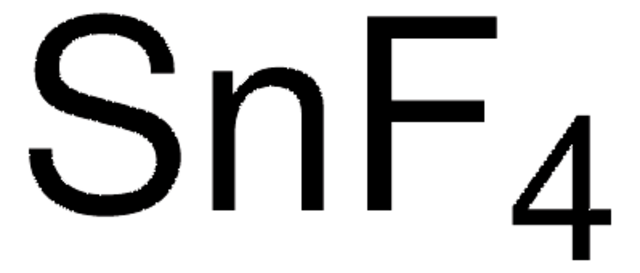The method of preparation for these products is considered proprietary; however, both items are manufactured from the same synthesized precursor. Each item is prepared using a unique method to meet specifications, i.e., powder or beaded form, purity. Neither are additionally purified.
所有图片(1)
选择尺寸
变更视图
1 G
$239.00
About This Item
线性分子式:
SnI2
CAS号:
分子量:
372.52
EC 号:
MDL编号:
UNSPSC代码:
12352302
PubChem化学物质编号:
NACRES:
NA.23
推荐产品
质量水平
方案
99.999% trace metals basis
表单
powder
杂质
≤15.0 ppm Trace Metal Analysis
沸点
714 °C (lit.)
mp
320 °C (lit.)
密度
5.28 g/mL at 25 °C (lit.)
SMILES字符串
I[SnH2]I
InChI
1S/2HI.Sn/h2*1H;/q;;+2/p-2
InChI key
JTDNNCYXCFHBGG-UHFFFAOYSA-L
正在寻找类似产品? 访问 产品对比指南
一般描述
碘化锡(II)是一种橙色结晶固体,熔点高。广泛用于制造有机–无机太阳能电池、发光器件、二次电池等。起还原剂的作用。
警示用语:
Danger
危险分类
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT RE 2 - STOT SE 3
靶器官
Cardio-vascular system,hematopoietic system, Respiratory system
储存分类代码
8A - Combustible corrosive hazardous materials
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
Water-stable 1D hybrid tin (II) iodide emits broad light with 36\% photoluminescence quantum efficiency
Ioannis Spanopoulos, et al.
Journal of the American Chemical Society, 142, 9028-9038 (2020)
Enhancing performance and stability of tin halide perovskite light emitting diodes via coordination engineering of lewis acid--base adducts
Ye Jin Heo, et al.
Advanced Functional Materials
, 31, 210697-210697 (2021)
Solvent-mediated crystallization of CH3NH3SnI3 films for heterojunction depleted perovskite solar cells
Feng Hao, et al.
Journal of the American Chemical Society, 137, 11445-11452 (2015)
A cation-exchange approach for the fabrication of efficient methylammonium tin iodide perovskite solar cells
Fengzhu Li, et al.
Angewandte Chemie (International Edition in English), 58, 6688-6692 (2019)
All-perovskite tandem solar cells with 24.2\% certified efficiency and area over 1 cm2 using surface-anchoring zwitterionic antioxidant
Ke Xiao, et al.
Nature Energy, 5, 870-880 (2020)
-
This is important to whether I buy your product.I would like to ask whether 409308 and 466352 are products obtained by two different synthetic routes or 466352 was obtained by further purifying 409308. And how do you synthesize and purify Tin(II) iodide?
1 answer-
Helpful?
-
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持