所有图片(2)
About This Item
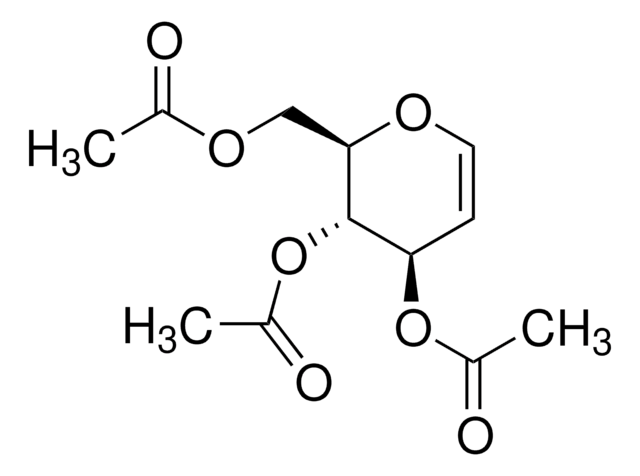
经验公式(希尔记法):
C6H10O4
CAS号:
分子量:
146.14
Beilstein:
81690
EC號碼:
MDL號碼:
分類程式碼代碼:
12352201
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
95%
光學活性
[α]22/D −21.5°, c = 1.2 in methanol
mp
99-103 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
OC[C@H]1OC=C[C@@H](O)[C@H]1O
InChI
1S/C6H10O4/c7-3-5-6(9)4(8)1-2-10-5/h1-2,4-9H,3H2/t4-,5-,6-/m1/s1
InChI 密鑰
YVECGMZCTULTIS-HSUXUTPPSA-N
應用
可同时用于寡糖的溶液相和固相合成的重要结构单元。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Affinity labelling of beta-D-galactosidase from Escherichia coli with D-[6-(3)H]-galactal.
G Kurz et al.
Carbohydrate research, 93(1), C14-C20 (1981-06-16)
Alessandra Bartolozzi et al.
The Journal of organic chemistry, 68(22), 8529-8533 (2003-10-25)
A nonclassical, totally stereoselective synthesis of orthogonally protected 1,3-disaccharides is reported. Enantiomerically pure beta-keto-delta-lactones, efficiently obtained from glucal and galactal, are transformed into electron-poor heterodienes and chemo-, regio-, and stereoselectively cycloadded to glycals as electron-rich dienophiles, to directly afford 2-thiodisaccharides.
Y X Lian et al.
Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chinese journal of integrated traditional and Western medicine, 21(9), 649-651 (2003-02-11)
To observe the effect of Shuangcao Tuihuang Granule-1 (SCTH-1) in treating severe jaundice of acute icterohepatitis and to study its mechanism. Thirty-four patients with severe jaundice of acute icterohepatitis were treated with SCTH-1, their therapeutic effects were analyzed. In the
Ileana Frau et al.
Chirality, 23(9), 820-826 (2011-12-03)
A convenient method for the stereoselective synthesis of diasteroisomeric vinyl epoxides (-)-2α and (-)-2β, the carba analogs of D-galactal and D-allal-derived vinyl epoxides 1α and 1β, has been elaborated starting from tri-O-acetyl-D-glucal. The key step of this synthesis is an
L Kiss et al.
Carbohydrate research, 291, 43-52 (1996-09-23)
C-(2-Deoxy-D-lyxo-hex-1-enopyranosyl)formamide was prepared from acetylated C-(beta-D-galactopyranosyl)formamide by a radical-mediated bromination-zinc/N-methylimidazole-induced reductive elimination-Zemplén deacetylation reaction sequence. The preparation of acetylated 5-(2-deoxy-D-lyxo-hex-1-enopyranosyl)tetrazole was improved. Methyl C-(2-deoxy-D-lyxo-hex-1-enopyranosyl)formimidate was transformed by benzylamine into N-benzyl-C-(2-deoxy-D-lyxo-hex-1-enopyranosyl)formamidine and, after hydrolysis to methyl C-(2-deoxy-D-lyxo-hex-1-enopyranosyl)formate, into N-benzyl-C-(2-deoxy-D-lyxo-hex-1-enopyranosyl)formamide. A series
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门