所有图片(1)
About This Item
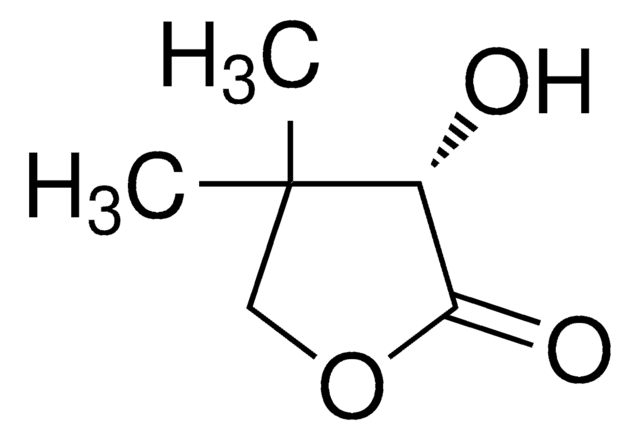
经验公式(希尔记法):
C4H6O3
CAS号:
分子量:
102.09
Beilstein:
80588
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
95%
形狀
liquid
光學活性
[α]23/D +66°, c = 1.15 in chloroform
光學純度
ee: 98% (GLC)
折射率
n20/D 1.467 (lit.)
bp
133 °C/10 mmHg (lit.)
密度
1.309 g/mL at 25 °C (lit.)
SMILES 字串
O[C@@H]1CCOC1=O
InChI
1S/C4H6O3/c5-3-1-2-7-4(3)6/h3,5H,1-2H2/t3-/m1/s1
InChI 密鑰
FWIBCWKHNZBDLS-GSVOUGTGSA-N
應用
(R)-(+)-α-Hydroxy-γ-butyrolactone can be used as a starting material to synthesize:
- δ-Azaproline by reacting with benzyloxycarbonyl aminophthalimide via Mitsunobu reactions.
- Homochiral (R)-2,4-dihydroxybutyramide seco-pseudonucleoside reagents.
- Botryolide B via esterification and ring-closing metathesis reaction.
- Pregnane derivatives containing γ-butyrolactones as potential glucocorticoid agonists.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves
Novel glucocorticoid antedrugs possessing a 21-(?-lactone) ring.
Angell RM, et al.
Journal of the Chemical Society. Perkin Transactions 1, 6, 831-839 (2002)
Concise total synthesis of botryolide B
Mohapatra DK, et al.
Royal Society of Chemistry Advances, 4(16), 8335-8340 (2014)
Novel glucocorticoid antedrugs possessing a 21-(γ-lactone) ring
Angell RM, et al.
Journal of the Chemical Society. Perkin Transactions 1, 4(6), 831-839 (2002)
Xiaohui Gou et al.
Frontiers in physiology, 11, 686-686 (2020-07-17)
Dentin sialoprotein (DSP), the NH2-terminal fragment of dentin sialophosphoprotein (DSPP), is essential for dentin formation and further processed into small fragments inside the odontoblasts. Gelatinases, including matrix metalloproteinases 9 (MMP9) and MMP2, were able to cleave DSP(P) in tooth structures.
Efficient synthesis of enantiomerically pure (S)-d-azaproline starting from (R)-a-hydroxy-?-butyrolactone via the Mitsunobu reaction.
Voss E, et al.
Tetrahedron Asymmetry, 20(15), 1809-1812 (2009)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门