所有图片(1)
About This Item
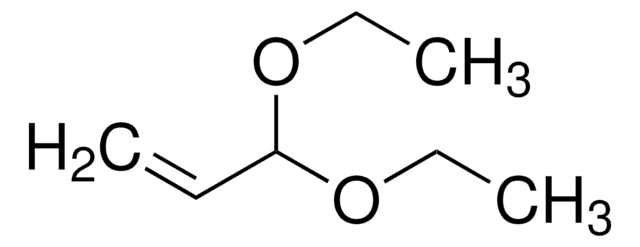
线性分子式:
CH3CH=CHCH(OC2H5)2
CAS号:
分子量:
144.21
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
等级
technical
方案
90%
折射率
n20/D 1.412 (lit.)
沸点
146-150 °C (lit.)
密度
0.831 g/mL at 25 °C (lit.)
SMILES字符串
[H]\C(C)=C(\[H])C(OCC)OCC
InChI
1S/C8H16O2/c1-4-7-8(9-5-2)10-6-3/h4,7-8H,5-6H2,1-3H3/b7-4+
InChI key
ZUMISMXLQDKQDS-QPJJXVBHSA-N
一般描述
trans-2-Butenal diethyl acetal is an acetal. Acetals are usually derived from the aldehydes. They have two single-bonded oxygen atoms attached to the same carbon atom. They are stable under basic conditions and they can be hydrolyzed back to the carbonyl compound. Acetals serves as a carbonyl protecting group and also are present as functional groups in glycosidic bonds. trans-2-Butenal diethyl acetal has been synthesized from trans-2-butenal with ethanol in the presence of methanesulfonic acid or methylsulfonate as catalysts.[1]
应用
trans-2-Butenal diethyl acetal may be used in the preparation of 2S,3S-epoxybutan-1-ol, an intermediate in the synthesis of erythromycin antibiotic.[2]
警示用语:
Warning
危险分类
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
3 - Flammable liquids
WGK
WGK 3
闪点(°F)
93.2 °F - closed cup
闪点(°C)
34 °C - closed cup
个人防护装备
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
An Efficient Method for One-Pot Reductive Cleavage of Acetals to Primary Alcohols Using a Bimetal Redox Couple CoCl2. 6H2O-Zn.
Sarma K and Goswami A.
Letters in Organic Chemistry, 6(7), 568-572 (2009)
SYNTHESIS OF trans-2-BUTENAL DIETHYL ACETAL CATALYZED BY METHYLSUFONIC ACID OR METHYLSULFONATE [J].
Lifang Z, et al.
Speciality Petrochemicals, 3, 008-008 (2006)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门