推荐产品
方案
97%
旋光性
[α]23/D −146°, c = 1 in chloroform
mp
82-85 °C (lit.)
SMILES字符串
FC(F)(F)S(=O)(=O)Oc1ccc2ccccc2c1-c3c(OS(=O)(=O)C(F)(F)F)ccc4ccccc34
InChI
1S/C22H12F6O6S2/c23-21(24,25)35(29,30)33-17-11-9-13-5-1-3-7-15(13)19(17)20-16-8-4-2-6-14(16)10-12-18(20)34-36(31,32)22(26,27)28/h1-12H
InChI key
OYJLCOSEYYZULE-UHFFFAOYSA-N
应用
(R)-(−)-1,1′-Bi-2-naphthol bis(trifluoromethanesulfonate) can be used:
- In the synthesis of heterobidentate ligands for asymmetric catalysis.[1][2]
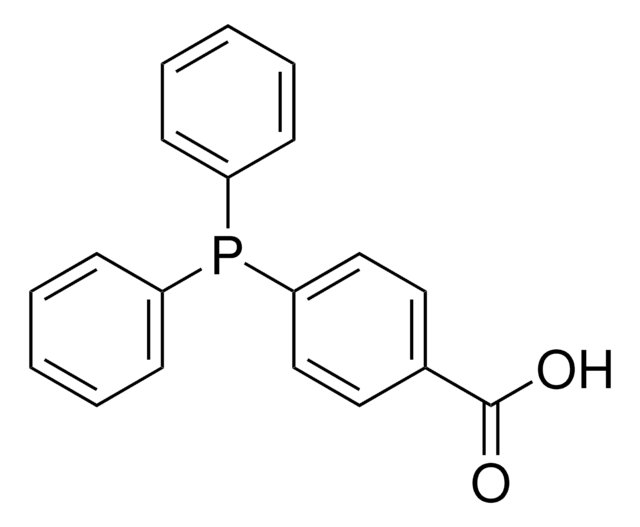
- To prepare 2-(diphenylphosphino)-2′-alkoxy-1,1′-binaphthyls by reacting with bis(aryl) phosphonic acids.[3]
- As a starting material for the synthesis of binaphthyl based rhodium catalysts used in the hydrogenation of styrene.[4]
警示用语:
Danger
危险声明
危险分类
Skin Corr. 1B
储存分类代码
8A - Combustible corrosive hazardous materials
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Synthesis of chiral 2, 2′-bis (diphenylphosphino)-1, 1′-binaphthyl (BINAP) via a novel nickel-catalyzed phosphine insertion
Cai D, et al.
The Journal of Organic Chemistry, 59(23), 7180-7181 (1994)
Palladium-catalysed asymmetric allylic alkylation in the presence of a chiral `light fluorous? phosphine ligand
Cavazzini M, et al.
Chemical Communications (Cambridge, England), 13, 1220-1221 (2001)
New perfluorinated rhodium-BINAP catalysts and hydrogenation of styrene in supercritical CO2
Altinel H, et al.
Journal of Supercritical Fluids, 51(2), 202-208 (2009)
Novel heterobidentate ligands for asymmetric catalysis: Synthesis and rhodium-catalysed reactions of S-alkyl (R)-2-diphenylphosphino-1, 1′-binaphthyl-2′-thiol
Gladiali S, et al.
Tetrahedron Asymmetry, 5(7), 1143-1146 (1994)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持![2,6-双[(4S)-(-)-异丙基-2-噁唑啉-2-基]吡啶 99%](/deepweb/assets/sigmaaldrich/product/structures/452/550/7e22a7c6-e84a-4741-af9a-e40f05d8061c/640/7e22a7c6-e84a-4741-af9a-e40f05d8061c.png)
![2,6-双[(4S)-4-苯基-2-噁唑啉基]吡啶 98%](/deepweb/assets/sigmaaldrich/product/structures/372/262/fb5c79fe-8277-48b0-a73e-4124c7c2c41c/640/fb5c79fe-8277-48b0-a73e-4124c7c2c41c.png)


![2,2′-异亚丙基双[(4S)-4-叔丁基-2-噁唑啉] 99%](/deepweb/assets/sigmaaldrich/product/structures/334/357/19788a81-5365-46fd-978b-6b98382b1117/640/19788a81-5365-46fd-978b-6b98382b1117.png)
![2,6-双[(4R)-(+)-异丙基-2-噁唑啉-2-基]吡啶 99%](/deepweb/assets/sigmaaldrich/product/structures/349/609/8673c46e-368a-47a6-a9bd-52bbe13d490a/640/8673c46e-368a-47a6-a9bd-52bbe13d490a.png)

![2,6-双[(3aR,8aS)-(+)-8H-茚并[1,2-d]噁唑啉-2-基)吡啶 ≥94%](/deepweb/assets/sigmaaldrich/product/structures/123/619/565288e2-e1c9-4825-a440-17e786bc2c27/640/565288e2-e1c9-4825-a440-17e786bc2c27.png)
![(-)-2,2′-异亚丙基双[(4S)-4-苯基-2-噁唑啉] 97%](/deepweb/assets/sigmaaldrich/product/structures/297/720/a29f61c3-34e4-410c-acdd-241699b80af3/640/a29f61c3-34e4-410c-acdd-241699b80af3.png)