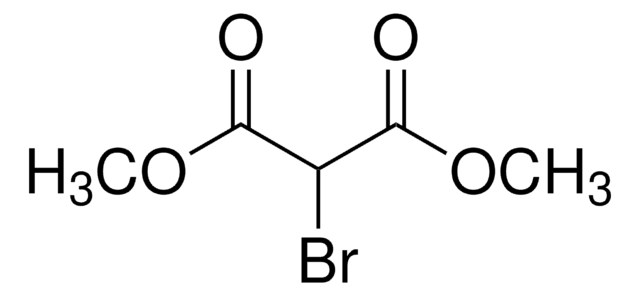
所有图片(1)
选择尺寸
变更视图
5 ML
$135.00
25 ML
$380.00
About This Item
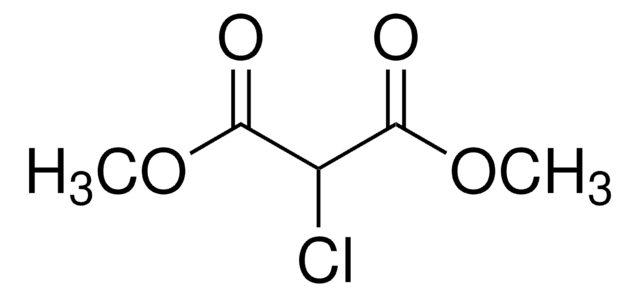
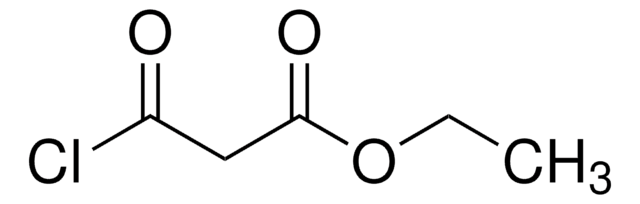
线性分子式:
ClCH(CO2C2H5)2
CAS号:
分子量:
194.61
EC 号:
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
95%
表单
liquid
折射率
n20/D 1.432 (lit.)
密度
1.204 g/mL at 25 °C (lit.)
官能团
chloro
ester
SMILES字符串
CCOC(=O)C(Cl)C(=O)OCC
InChI
1S/C7H11ClO4/c1-3-11-6(9)5(8)7(10)12-4-2/h5H,3-4H2,1-2H3
InChI key
WLWCQKMQYZFTDR-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
Diethyl chloromalonate (Diethyl α-chloromalonate) is a 2-halo-1,3-dicarbonyl compound.[1] It participates in K2CO3-catalyzed domino reactions (Michael alkylation, Mannich alkylation, and aldol alkylation) of salicylic aldehyde derivatives to afford functionalized 2,3-dihydrobenzofurans.[2] It reacts with Cs2CO3 in the presence of elemental S8 or Sen to afford the corresponding diethyl thioxo- or selenoxomalonates, which can be trapped in situ with various 1,3-dienes.[3]
警示用语:
Danger
危险声明
危险分类
Aquatic Acute 1 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
靶器官
Respiratory system
储存分类代码
8A - Combustible corrosive hazardous materials
WGK
WGK 3
闪点(°F)
235.4 °F - closed cup
闪点(°C)
113 °C - closed cup
个人防护装备
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
R N Henrie et al.
Journal of medicinal chemistry, 26(4), 559-563 (1983-04-01)
Reaction of diethyl chloromalonate with beta-mercapto amines, 9, gave 1,4-thiazin-3-ones, 10, which were alkylated exclusively at the lactam oxygen with triethyloxonium tetrafluoroborate and subsequently condensed with guanidine to give the first reported 5-thiapterins, 8. Oxidation of 8 with m-chloroperoxybenzoic acid
Preparation of cycloaddition chemistry of thio-and selenocarbonyls derived from reaction of elemental sulfur and selenium with stabilized a-halo anions.
Abelman MM.
Tetrahedron Letters, 32(50), 7389-7392 (1990)
Qu-Bo Li et al.
The Journal of organic chemistry, 76(17), 7222-7228 (2011-07-29)
The K(2)CO(3)-catalyzed domino reactions (Michael alkylation, Mannich alkylation, and aldol alkylation) of salicylic aldehyde derivatives (2-hydroxyaryl-α,β-unsaturated ketones, 2-hydroxyarylnitroalkenes, 2-hydroxyarylimines, and salicylic aldehydes) and 2-halo-1,3-dicarbonyl compounds (diethyl α-bromomalonate, diethyl α-chloromalonate, ethyl 2-chloroacetoacetate, and 3-chloropentane-2,4-dione) were carried out under mild conditions to
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门