所有图片(1)
选择尺寸
变更视图
25 G
$95.20
100 G
$211.00
About This Item
经验公式(希尔记法):
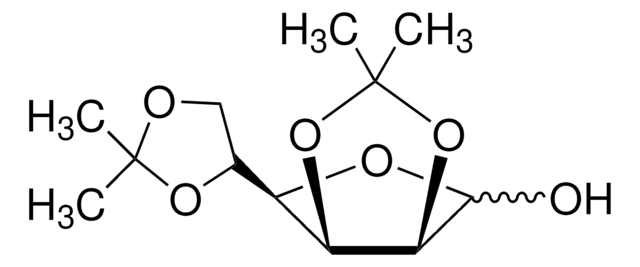
C12H20O6
CAS号:
分子量:
260.28
Beilstein:
84386
EC 号:
MDL编号:
UNSPSC代码:
12352201
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
等级
purum
方案
≥98.0% (TLC)
旋光性
[α]20/D −11.5±1°, c = 5% in ethanol
mp
110-111 °C (lit.)
SMILES字符串
CC1(C)OC[C@@H](O1)[C@H]2O[C@@H]3OC(C)(C)O[C@@H]3[C@H]2O
InChI
1S/C12H20O6/c1-11(2)14-5-6(16-11)8-7(13)9-10(15-8)18-12(3,4)17-9/h6-10,13H,5H2,1-4H3/t6-,7+,8-,9-,10-/m1/s1
InChI key
KEJGAYKWRDILTF-JDDHQFAOSA-N
正在寻找类似产品? 访问 产品对比指南
储存分类代码
11 - Combustible Solids
WGK
WGK 2
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
S. Iacono et al.
Organic Syntheses, 64, 57-57 (1986)
Yoon-Suk Kang et al.
Infection and immunity, 87(8) (2019-06-05)
Brucella is an intracellular bacterial pathogen that causes chronic systemic infection in domesticated livestock and poses a zoonotic infectious risk to humans. The virulence of Brucella is critically dependent on its ability to replicate and survive within host macrophages. Brucella
Guylaine M Defossemont et al.
Carbohydrate research, 338(6), 563-565 (2003-04-02)
The synthesis and characterisation of a novel chiral bicyclic oxacaprolactone is reported. The choice of diisopropylidene-D-glucose as a starting material allowed selective introduction of the synthetic equivalent necessary for the formation of the seven-membered ring of the lactone, i.e., one
S C Hung et al.
Carbohydrate research, 331(4), 369-374 (2001-06-12)
A practical route toward the synthesis of 6-deoxy-L-idose and L-acovenose from 1,2:5,6-di-O-isopropylidene-alpha-D-glucofuranose is described. Key steps include the stereoselective hydrogenation of 6-deoxy-1,2:3,5-di-O-isopropylidene-alpha-D-xylo-hex-5-enofuranose, regioselective protection of 6-deoxy-1,2-O-isopropylidene-beta-L-idofuranose at 0-5, and epimerisation of 6-deoxy-5-O-tert-butyldimethylsilyl-1,2-O-isopropylidene-beta-L-idofuranose at C-3.
Z Huang et al.
Methods in molecular biology (Clifton, N.J.), 20, 315-353 (1993-01-01)
Two sets of experimental protocols are given for the synthesis of 3',5'-bis-homodeoxyribonucleosides, building blocks for the synthesis of oligodeoxynucleotide analogs where the -O-PO2-O- groups are replaced by -CH2-S-CH2-, -CH2-SO-CH2-, and -CH2-SO2-CH2- units. Conditions are presented for joining these building blocks
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![(3AR,5S,6S,6AR)-5-((R)-2,2-DIMETHYL-1,3-DIOXOLAN-4-YL)-2,2-DIMETHYLTETRAHYDROFURO[3,2-D][1,3]DIOXOL-6-OL AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/241/825/1c695a85-5c36-42d3-806a-30876a4dabac/640/1c695a85-5c36-42d3-806a-30876a4dabac.png)