所有图片(1)
选择尺寸
变更视图
1 G
$61.70
5 G
$252.00
About This Item
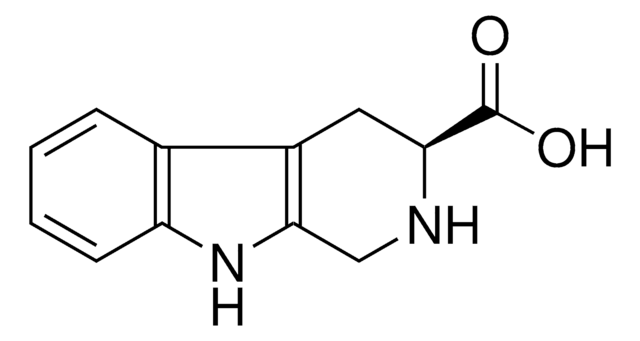
经验公式(希尔记法):
C11H12N2
CAS号:
分子量:
172.23
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
98%
表单
liquid
mp
206-208 °C (lit.)
SMILES字符串
C1Cc2c(CN1)[nH]c3ccccc23
InChI
1S/C11H12N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-4,12-13H,5-7H2
InChI key
CFTOTSJVQRFXOF-UHFFFAOYSA-N
基因信息
rat ... Htr2a(29595) , Htr2c(25187)
一般描述
Ozonolysis of the enamine bond of 1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole derivatives was studied.[1]
应用
- Reactant for synthesis of the indolyl-β-carboline alkaloid eudistomin U via IBX mediated room temperature oxidative aromatization
- Reactant for preparation of neuroprotective HDAC6 inhibitors
- Reactant for preparation of aminofuranopyrimidines as EGFR and Aurora A kinase inhibitors
- Reactant for preparation of inhibitors of CDK4
- Reactant for preparation of tetrahydrocarboline derivatives of as human 5-HT5A receptor ligands
- Reactant for preparation of 5-(diaminomethyl)-2,4-aminopyrimidines as dihydrofolate reductase inhibitors and antibacterial agents
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
Minoru Tanaka et al.
Bioorganic & medicinal chemistry, 21(5), 1159-1165 (2013-01-23)
Indoleamine 2,3-dioxygenase (IDO) plays a significant role in several disorders such as Alzheimer's disease, age-related cataracts and tumors. A series of novel tryptoline derivatives were synthesized and evaluated for their inhibitory activity against IDO. Substituted tryptoline derivatives (11a, 11c, 11e
Takayoshi Arai et al.
The Journal of organic chemistry, 76(8), 2909-2912 (2011-03-08)
A four-step synthetic route to fully substituted chiral tetrahydro-β-carbolines (THBCs) is described. Starting from the (R,S,S)-Friedel-Crafts/Henry adduct obtained from three-component coupling of an indole, nitroalkene, and aldehyde catalyzed by imidazoline-aminophenol-CuOTf, the (1S,3S,4R)-THBCs were readily synthesized in a three-step operation including
I M McDonald et al.
Journal of medicinal chemistry, 43(19), 3518-3529 (2000-09-23)
A novel series of nonpeptide CCK(2) receptor antagonists has been prepared, in which 2,7-dioxo-2,3,4,5,6,7-hexahydro-1H-benzo[h][1, 4]diazonine (5) was used as a chemical template. This uncommon ring system was obtained in a highly substituted form and in high yield by ozonolysis of
Nermin S Ahmed et al.
Archiv der Pharmazie, 344(3), 149-157 (2011-03-09)
Starting from tadalafil as a template, a series of functionalized tetrahydro-β-carboline derivatives have been prepared and identified as novel potent and selective PDE5 inhibitors. Replacing the 3,4-methylenedioxyphenyl at position 6 of tadalafil, together with elongation of the N2-methyl substituent and
Jutamas Jiaranaikulwanitch et al.
Bioorganic & medicinal chemistry letters, 20(22), 6572-6576 (2010-10-15)
Tryptoline, a core structure of ochrolifuanine E, which is a hit compound from virtual screening of the Thai herbal database against BACE1 was used as a scaffold for the design of BACE1 inhibitors. The tryptoline was linked with different side
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持


![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/376/664/07577eb6-6e8c-4237-b8c5-03da4c8e7d88/640/07577eb6-6e8c-4237-b8c5-03da4c8e7d88.png)