所有图片(1)
选择尺寸
变更视图
5 G
$156.00
About This Item
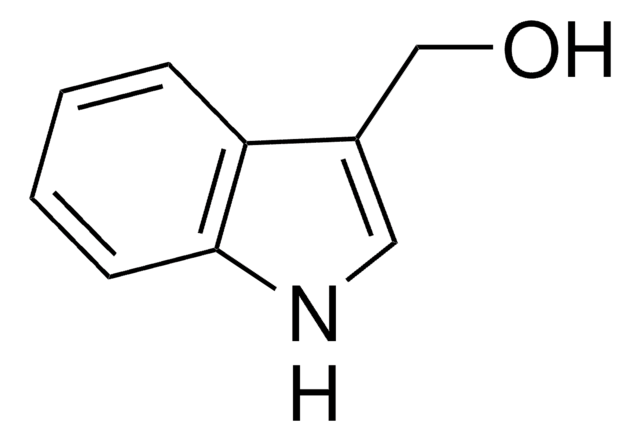
经验公式(希尔记法):
C9H7NO2
CAS号:
分子量:
161.16
Beilstein:
129435
EC 号:
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
产品线
ReagentPlus®
方案
99%
表单
solid
mp
232-234 °C (dec.) (lit.)
溶解性
95% ethanol: soluble 5%, clear to slightly hazy, light yellow to yellow
SMILES字符串
OC(=O)c1c[nH]c2ccccc12
InChI
1S/C9H7NO2/c11-9(12)7-5-10-8-4-2-1-3-6(7)8/h1-5,10H,(H,11,12)
InChI key
KMAKOBLIOCQGJP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
吲哚-3-羧酸衍生物的结构已采用高分辨质谱气相色谱(GC-HRMS)、超高效液相色谱结合高分辨率串联质谱(UHPLC-HRMS)、核磁共振光谱(NMR)和傅里叶变换红外光谱(FT-IR)进行了研究[1]。
应用
作为反应物用于制备:
- 抗癌剂
- 氨基酸和肽的衍生物
- 5-羟色胺5-HT4受体拮抗剂
- 主要的酰基脲类
- 在Hedgehog途径中,Gli1介导的转录的抑制剂
- 5-羟色胺5-HT6拮抗剂
- 超晚期抗原-4(VLA-4)拮抗剂
- EphB3受体酪氨酸激酶抑制剂
- 阿尔茨海默病的潜在治疗药物
- 乙烯基酯假三肽蛋白酶体抑制剂
法律信息
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
其他客户在看
Xuejin Zhao et al.
Viruses, 13(8) (2021-08-29)
Influenza A viruses are serious zoonotic pathogens that continuously cause pandemics in several animal hosts, including birds, pigs, and humans. Indole derivatives containing an indole core framework have been extensively studied and developed to prevent and/or treat viral infection. This
Analytical characterization of some synthetic cannabinoids, derivatives of indole-3-carboxylic acid.
Vadim Shevyrin et al.
Forensic science international, 232(1-3), 1-10 (2013-09-24)
By means of gas chromatography with high resolution mass spectrometry (GC-HRMS), ultra-high performance liquid chromatography in combination with high resolution tandem mass spectrometry (UHPLC-HRMS), nuclear magnetic resonance spectroscopy (NMR) and Fourier transform infrared spectroscopy (FT-IR), structure of a series from
Mu-Yang Wang et al.
Journal of integrative plant biology, 54(7), 471-485 (2012-05-26)
Camalexin (3-thiazol-2'-yl-indole) is the major phytoalexin found in Arabidopsis thaliana. Several key intermediates and corresponding enzymes have been identified in camalexin biosynthesis through mutant screening and biochemical experiments. Camalexin is formed when indole-3-acetonitrile (IAN) is catalyzed by the cytochrome P450
M U Ahmad et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 23(9), 841-847 (1985-09-01)
The nitrosation of gramine, a tertiary amine alkaloid present in barley malt, was carried out by reaction with sodium nitrite in buffered acetic acid (pH 3.4) for 1 hr at room temperature. Two major non-volatile products of the nitrosation reaction
Jin-Mo Ku et al.
The Journal of organic chemistry, 72(21), 8115-8118 (2007-09-20)
An enantioselective synthetic method for (-)-cis-clavicipitic acid (1) was reported. 1 was obtained in 10 steps (99% ee and 20% overall yield) from 1H-indole-3-carboxylic acid methyl ester (9) via asymmetric phase-transfer catalytic alkylation and diastereoselective Pd(II)-catalyzed intramolecular aminocyclization as key
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门