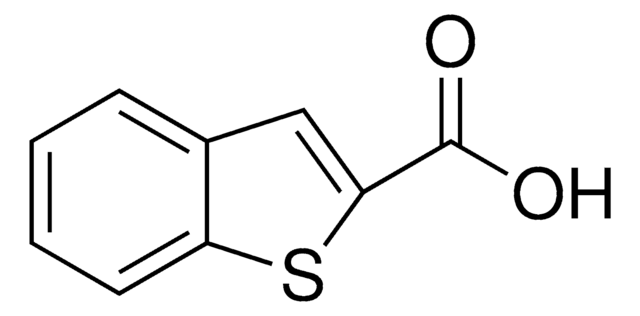
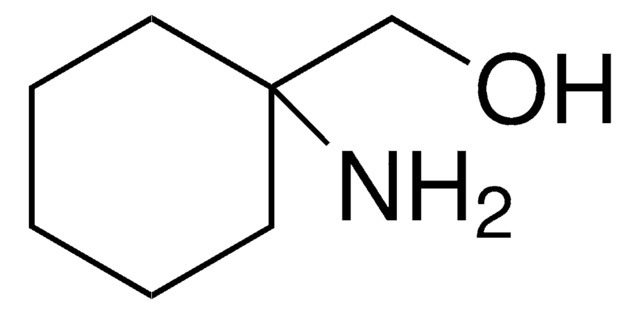
推荐产品
化驗
98%
形狀
solid
反應適用性
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
應用
peptide synthesis
SMILES 字串
NC1(CCCCC1)C(O)=O
InChI
1S/C7H13NO2/c8-7(6(9)10)4-2-1-3-5-7/h1-5,8H2,(H,9,10)
InChI 密鑰
WOXWUZCRWJWTRT-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
A Romanelli et al.
Journal of peptide science : an official publication of the European Peptide Society, 7(1), 15-26 (2001-03-14)
Secondary structure formation and stability are essential features in the knowledge of complex folding topology of biomolecules. To better understand the relationships between preferred conformations and functional properties of beta-homo-amino acids, the synthesis and conformational characterization by X-ray diffraction analysis
Wioleta Kowalczyk et al.
Journal of medicinal chemistry, 47(24), 6020-6024 (2004-11-13)
The synthesis and some pharmacological properties of two sets of analogues, one consisting of six peptides with 1-aminocyclohexane-1-carboxylic acid (Acc) in position 2 and the other with the amino acid in position 3, have been described. All the peptides were
Fernando Formaggio et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 8(1), 84-93 (2002-02-02)
The achiral, nitroxyl-containing alpha-amino acid TOAC (TOAC = 2,2,6,6-tetramethylpiperidine-1-oxyl-4-amino-4-carboxylic acid), in combination with the chiral alpha-amino acid C(alpha)-methyl valine [(alphaMe)Val], was used to prepare short peptides (from di- to hexa-) that induced the enantioselective oxidation of racemic 1-phenylethanol to acetophenone.
Mitsunobu Doi et al.
Biochemical and biophysical research communications, 297(1), 138-142 (2002-09-11)
Endomorphin (EM2, Tyr-Pro-Phe-Phe-NH(2)) can assume various conformations related to cis/trans-rotamers of the amide linkage of Tyr-Pro. To control isomerization, restricted or flexible components have been introduced at the Pro position. We focused on [Chx(2)]EM2, an EM2 analogue substituting 1-aminocyclohexane-1-carboxlylic acid
Olga Labudda-Dawidowska et al.
Journal of medicinal chemistry, 48(25), 8055-8059 (2005-12-13)
In the present work, a sterically constrained noncoded amino acid, 1-aminocyclohexane-1-carboxylic acid (Acc), was substituted in position 8 of the peptide chain of bradykinin (BK) and position 6, 7, or 8 of its B2 receptor antagonist [D-Arg0,Hyp3,Thi,(5,8)D-Phe7]BK, previously synthesized by
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门