推荐产品
化驗
≥98%
形狀
solid
mp
96-98 °C (lit.)
SMILES 字串
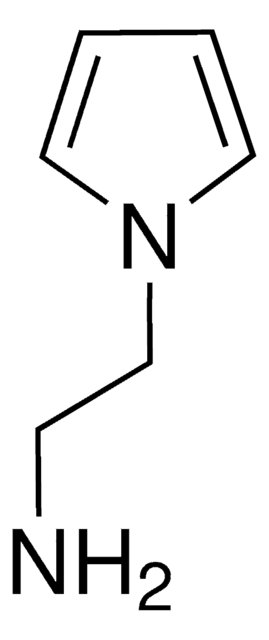
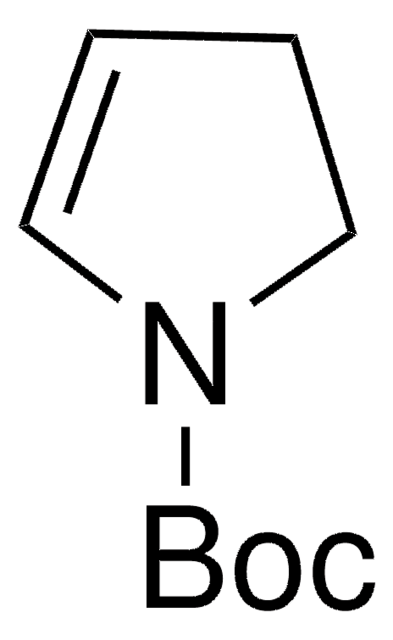
Nc1ccccc1-n2cccc2
InChI
1S/C10H10N2/c11-9-5-1-2-6-10(9)12-7-3-4-8-12/h1-8H,11H2
InChI 密鑰
GDMZHPUPLWQIBD-UHFFFAOYSA-N
一般說明
1-(2-Aminophenyl)pyrrole participates in Pt(IV)-catalyzed hydroamination triggered cyclization reaction to yield fused pyrrolo [1,2-a] quinoxalines. It reacts with aromatic or heteroaromatic aldehydes in ethanol and catalytic amounts of acetic acid to yield 4,5-dihydropyrrolo[1,2-a]quinoxalines. Thin films of poly(1-(2-aminophenyl)pyrrole) has been prepared via oxidative electropolymerization.
應用
1-(2-Aminophenyl)pyrrole was used in the synthesis of 4-substituted pyrrolo[1,2-a]quinoxaline derivatives.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Nitin T Patil et al.
The Journal of organic chemistry, 75(10), 3371-3380 (2010-04-16)
A PtCl(4)-catalyzed hydroamination-triggered cyclization strategy to access biologically interesting N-containing heterocycles such as pyrrolo[1,2-a]quinoxalines, indolo[1,2-a]quinoxalines, and indolo[3,2-c]quinolines is described. The reaction makes use of aminoaromatics such as 1-(2-aminophenyl)pyrroles, N-(2-aminophenyl)indoles, 2-(2-aminophenyl)indoles, and alkynes having a tethered hydroxyl group. Mechanistically, the reaction
Jean Guillon et al.
Bioorganic & medicinal chemistry, 15(1), 194-210 (2006-10-20)
An original series of 4-substituted pyrrolo[1,2-a]quinoxaline derivatives, new structural analogues of Galipea species quinoline alkaloids, was synthesized from various substituted 2-nitroanilines via multistep heterocyclizations and tested for in vitro antiparasitic activity upon Leishmania amazonensis and Leishmania infantum strains. Structure-activity relationships
Photoelectrochemical studies on poly [1-(2-aminophenyl) pyrrole]-Creation of a photoactive inorganic-organic semiconductor interface (IOI).
Kasem KK, et al.
Canadian Journal of Chemistry, 87(8), 1109-1116 (2009)
A Versatile Synthesis of 4, 5-Dihydropyrrolo [1, 2-a] quinoxalines.
Abonia R, et al.
Journal of Heterocyclic Chemistry, 38(3), 671-674 (2001)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门







![四[3,5-二(三氟甲基)苯基]硼酸钾 Selectophore™](/deepweb/assets/sigmaaldrich/product/structures/631/130/b5486f44-2e69-40d0-902f-dd71894a6add/640/b5486f44-2e69-40d0-902f-dd71894a6add.png)