所有图片(1)
About This Item
经验公式(希尔记法):
C6H10O2
CAS号:
分子量:
114.14
Beilstein:
1341205
EC 号:
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
95%
表单
liquid
折射率
n20/D 1.462 (lit.)
沸点
59 °C/20 mmHg (lit.)
密度
1.022 g/mL at 25 °C (lit.)
官能团
ether
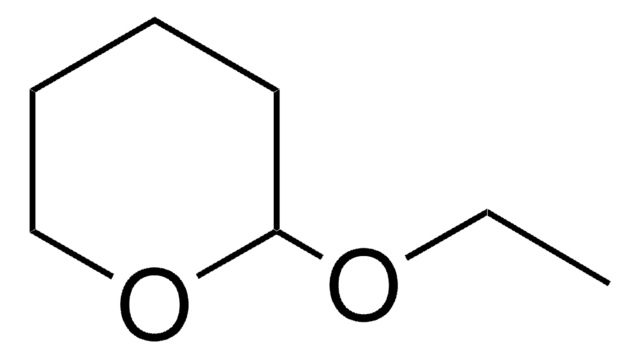
SMILES字符串
COC1=CCOCC1
InChI
1S/C6H10O2/c1-7-6-2-4-8-5-3-6/h2H,3-5H2,1H3
InChI key
FSMHNRHLQAABPS-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
5,6-Dihydro-4-methoxy-2H-pyran is suitable reagent for protection of nucleoside hydroxyl functions.
应用
用于手性醇保护的试剂。
A J Leigh et al.
Biochemistry, 31(37), 8978-8983 (1992-09-22)
The substrate stereospecificity of phosphatidylinositol-specific phospholipase C from Bacillus cereus is examined using the resolved optical isomers of synthetic myo-inositol 1-(4-nitrophenyl phosphate), a chromogenic substrate for the phospholipase. The synthetic route employs mild acid-labile protecting groups and separation of the
Tetrahedron Letters, 33, 2371-2371 (1992)
1, 1-Diethoxybut-2-ene as a Precursor of (2-Hydroxyethyl)-Substituted Alkoxy Dienes: Convenient Intermediates for a New Synthesis of 2-Substituted and 2, 6-Disubstituted Tetrahydro-4H-pyran-4-ones.
Prandi C and Venturello P.
The Journal of Organic Chemistry, 59(12), 3494-3496 (1994)
C Lehmann et al.
Nucleic acids research, 17(7), 2379-2390 (1989-04-11)
Efficient solid-phase synthesis of a series of oligoribonucleotides of up to 20 residues is described that utilises the 9-fluorenylmethoxycarbonyl group (Fmoc) for 5'-protection and 4-methoxytetrahydropyran-4-yl (Mthp) for 2'-protection of ribonucleotide monomers and a phosphoramidite coupling procedure. The Fmoc group is
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门