推荐产品
方案
99%
mp
122-124 °C (lit.)
SMILES字符串
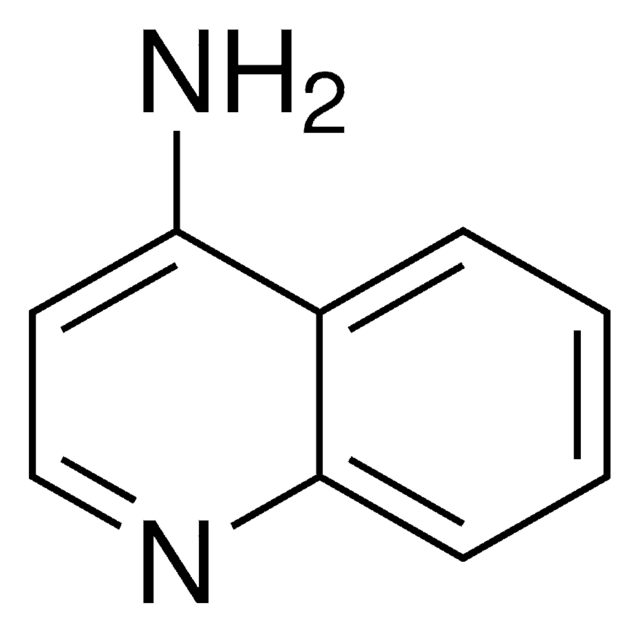
Nc1nccc2ccccc12
InChI
1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11)
InChI key
OSILBMSORKFRTB-UHFFFAOYSA-N
应用
1-Aminoisoquinoline was used in the synthesis of pyrimidoisoquinolinone[1].
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
Scott P Brown et al.
Journal of medicinal chemistry, 52(10), 3159-3165 (2009-04-24)
We apply a high-throughput formulation of the molecular mechanics with Poisson-Boltzmann surface area (htMM-PBSA) to estimate relative binding potencies on a set of 308 small-molecule ligands in complex with the proteins urokinase, PTP-1B, and Chk-1. We observe statistically significant correlation
J B Rewinkel et al.
Bioorganic & medicinal chemistry letters, 9(5), 685-690 (1999-04-14)
Replacement of the highly basic benzamidine moiety of NAPAP by the moderately basic 1-aminoisoquinoline moiety resulted in thrombin inhibitors with improved selectivity towards trypsin and enhanced Caco-2 cell permeability.
Hervé Bibas et al.
The Journal of organic chemistry, 67(8), 2619-2631 (2002-04-13)
The synthesis, spectroscopic properties, and chemical reactions of the stable (neopentylimino)-, (mesitylimino)-, and (o-tert-butylphenylimino)propadienones (6) are reported. Nucleophilic addition of amines affords the malonic amidoamidines 7 and 8. 3,5-Dimethylpyrazole reacts analogously to form 9b. Addition of 1,2-dimethylhydrazine produces pyrazolinones 10-12.
Xiaohong Wei et al.
Organic letters, 13(17), 4636-4639 (2011-08-05)
[RhCp*Cl(2)](2) can catalyze the oxidative coupling of N-aryl and N-alkyl benzamidines with alkynes to give N-substituted 1-aminoisoquinolines in high selectivity.
[A practical method for the synthesis of 1-aminoisoquinoline].
S Giorgi-Renault et al.
Annales pharmaceutiques francaises, 41(6), 555-557 (1983-01-01)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持