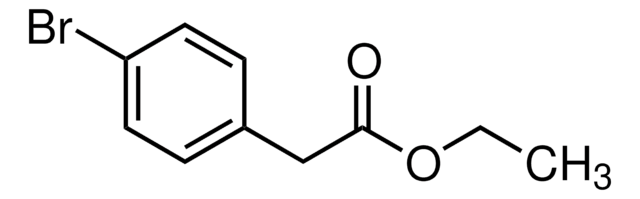
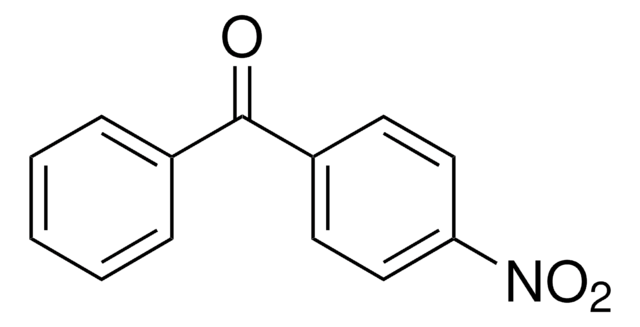
推荐产品
方案
98%
表单
powder
mp
82-85 °C (lit.)
SMILES字符串
[O-][N+](=O)c1ccccc1CC#N
InChI
1S/C8H6N2O2/c9-6-5-7-3-1-2-4-8(7)10(11)12/h1-4H,5H2
InChI key
YPRFCQAWSNWRLM-UHFFFAOYSA-N
警示用语:
Warning
危险分类
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
Xiaofei Zhang et al.
Organic & biomolecular chemistry, 12(2), 355-361 (2013-11-23)
An effective and convenient method has been developed for the preparation of 2 or 2,4-substituted α-carbolines via a one-pot tandem reaction of α,β-unsaturated ketones with 2-nitrophenylacetonitrile in the presence of zinc dust, acetic acid and triethylamine. This protocol presents a
Takashi Ohshima et al.
Journal of the American Chemical Society, 124(49), 14546-14547 (2002-12-06)
The enantioselective total synthesis of (-)-strychnine was accomplished through the use of the highly practical catalytic asymmetric Michael reaction (0.1 mol % of (R)-ALB, more than kilogram scale, without chromatography, 91% yield and >99% ee) as well as a tandem
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持