157457
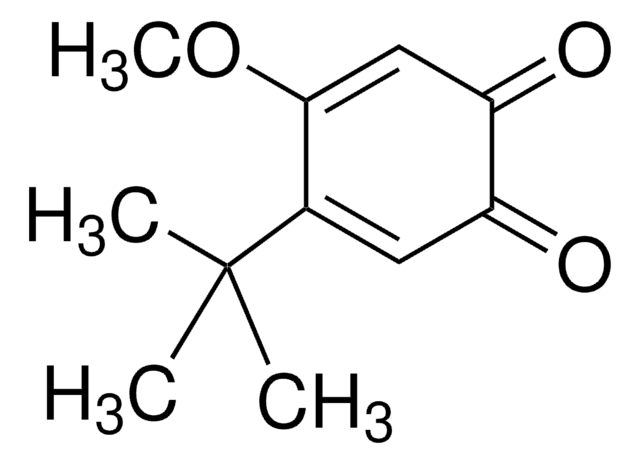
3,5-二叔丁基邻苯醌
98%
别名:
3,5-二叔丁基-1,2-苯醌, 3,5-二叔丁基-o-苯醌, 3,5-二叔丁基环己基-3,5-二烯-1,2-二酮, 3,5-二叔丁基苯醌, 3,5-双(1,1-二甲基乙基)-3,5-环己二烯-1,2-二酮, 4,6-二叔丁基-1,2-苯醌, 4,6-二叔丁基-o-苯醌
登录查看公司和协议定价
所有图片(1)
选择尺寸
变更视图
10 G
$343.00
About This Item
线性分子式:
[(CH3)3C]2C6H2(=O)2
CAS号:
分子量:
220.31
Beilstein:
2047944
EC 号:
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
质量水平
方案
98%
表单
solid
mp
112-114 °C (lit.)
SMILES字符串
CC(C)(C)C1=CC(=O)C(=O)C(=C1)C(C)(C)C
InChI
1S/C14H20O2/c1-13(2,3)9-7-10(14(4,5)6)12(16)11(15)8-9/h7-8H,1-6H3
InChI key
NOUZOVBGCDDMSX-UHFFFAOYSA-N
基因信息
human ... ACHE(43) , BCHE(590) , CES1(1066)
正在寻找类似产品? 访问 产品对比指南
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
M Angeles Alvarez et al.
Dalton transactions (Cambridge, England : 2003), 41(48), 14498-14513 (2012-10-12)
The title complexes reacted readily with the methylating agents MeI and CF(3)SO(3)Me, chalcogens (O(2), S(8)) and borane adducts BH(3)·L (L = THF, N(t)Bu(3), PPh(3)) to initially give the corresponding neutral or cationic derivatives of the type [Fe(2)Cp(2){μ-P(E)R}(μ-CO)(CO)(2)](n) (Cp = η(5)-C(5)H(5);
Hitomi Tanaka et al.
International journal of molecular sciences, 19(10) (2018-09-27)
It is generally considered that eumelanin (EM) is photoprotective while pheomelanin (PM) is phototoxic. A recent study using a mouse model demonstrated that PM produces reactive oxygen species (ROS) that cause DNA damage and eventually lead to melanomagenesis. A biochemical
Norma A Macías-Ruvalcaba et al.
The journal of physical chemistry. B, 110(43), 22043-22047 (2006-10-27)
The electrochemical reduction of 3,5-di-tert-butyl-1,2-benzoquinone, 1, has been studied in acetonitrile with added 2,2,2-trifluoroethanol, 2. At low concentrations of 2 the reaction proceeds by the following pathway: reduction of the quinone (Q) to its anion radical (Q*-) followed by complexation
Hetero Diels-Alder reactions of 3, 5-di-tert-butyl-o-benzoquinone with acyclic dienes: novel synthesis of 1, 4-benzodioxines.
Nair V and Kumar S.
Journal of the Chemical Society. Chemical Communications, 11, 1341-1342 (1994)
Olga Iasco et al.
Inorganic chemistry, 51(4), 2588-2596 (2012-01-12)
Two benzoxazoles derivative ligands were synthesized from the condensation of 3,5-di-tert-butyl-o-benzoquinone (DTBBQ) with ethanolamine or 1,3-diamino-2-hydroxypropane in methanol. Condensation of DTBBQ with ethanolamine gives the expected 5,7-di-tert-butyl-2-methylenhydroxylbenzoxazole (HL1) while with 1,3-diamino-2-hydroxypropane it gives (2-hydroxyethyl-2-{2,4-bis(1,1-dimethylethyl)-1-phenol-6 amino}-2{5,7-di-tert-butyl-benzoxazole}) (H(2)L2) with only one benzoxazole
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持