All Photos(1)
About This Item
Empirical Formula (Hill Notation):
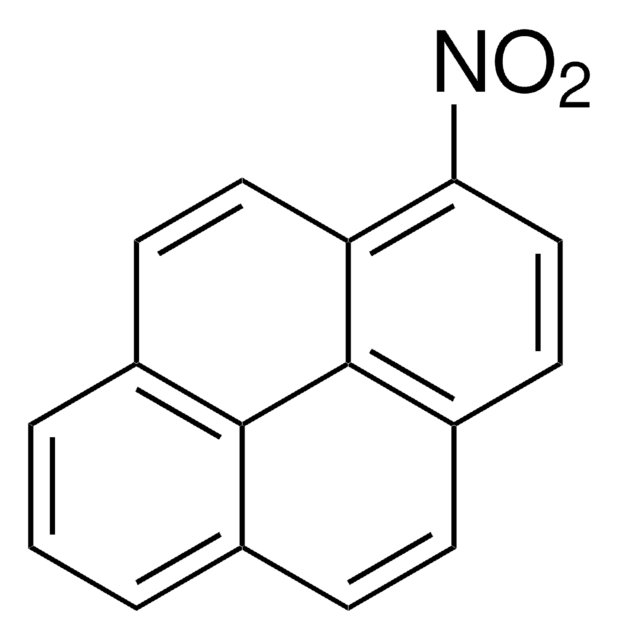
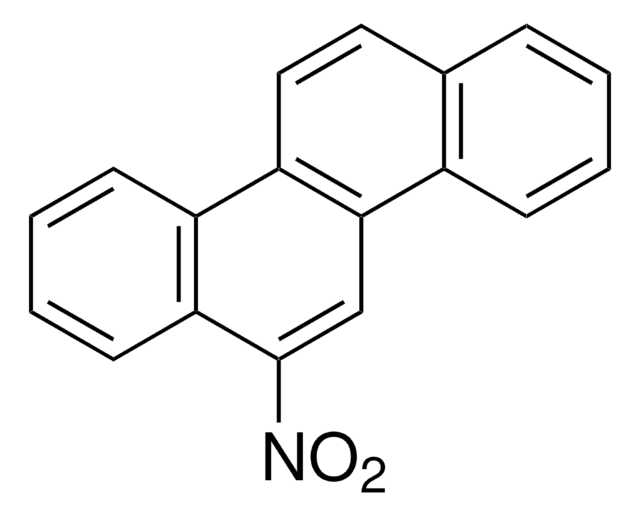
C14H9NO2
CAS Number:
Molecular Weight:
223.23
Beilstein:
1877509
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
93%
form
powder
mp
141-144 °C (lit.)
SMILES string
[O-][N+](=O)c1c2ccccc2cc3ccccc13
InChI
1S/C14H9NO2/c16-15(17)14-12-7-3-1-5-10(12)9-11-6-2-4-8-13(11)14/h1-9H
InChI key
LSIKFJXEYJIZNB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kazuyuki Mori et al.
Angewandte Chemie (International ed. in English), 54(23), 6847-6851 (2015-04-25)
A single-strand arylene-vinylene precursor containing four phenylene and three naphthylene units linked together with six vinylene spacers undergoes helical folding via sextuple photocyclization to give a [16]helicene core in a single step. The phenylene and naphthylene units are arranged in
Karla I Garfias-Gonzalez et al.
Molecules (Basel, Switzerland), 20(5), 8548-8559 (2015-05-20)
Two new classes of dendrimers bearing 8 and 32 fluorene donor groups have been synthesized. The first and second generations of these porphyrin-PAMAM-fluorene dendrimers were characterized by 1H-NMR, 13C-NMR, FTIR, UV-vis spectroscopy, elemental analyses and MALDI-TOF mass spectrometry. The UV-vis
B E Butterworth et al.
Mutagenesis, 16(2), 169-177 (2001-03-07)
Commercial anthraquinone (AQ) (9,10-anthracenedione) is produced by at least three different production methods worldwide: oxidation of anthracene (AQ-OX), Friedel-Crafts technology (AQ-FC) and by Diels-Alder chemistry (AQ-DA), with the final product varying in color and purity. AQ-OX begins with anthracene produced
Hiromichi Akizawa et al.
Chemical & pharmaceutical bulletin, 52(1), 41-46 (2004-01-08)
A comparative study was conducted to elucidate the mechanism underlying the separation of poly-aromatic-hydrocarbons (PAHs) and related compounds thereof on a column packed with silica gels modified with Ni(II)- or Cu(II)-phthalocyanine derivatives (PCS) (Ni- or Cu-PCS(D) column) and commercially available
M J Dennis et al.
Food additives and contaminants, 1(1), 29-37 (1984-01-01)
A method is described for the sample clean-up and estimation of nitropolycyclic aromatic hydrocarbons (nitro-PAH) in foods. The analysis involves the novel use of a coupled capillary gas chromatograph/thermal energy analyser and provides a detection limit for 1-nitropyrene of 12
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service