G6104
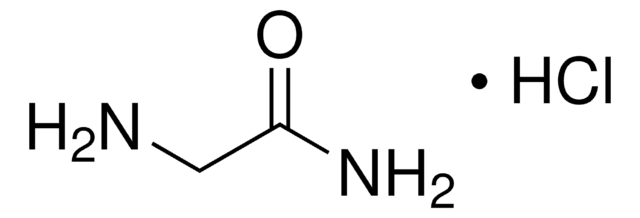
Glycinamide hydrochloride
98%
Synonym(s):
2-Aminoacetamide hydrochloride, Aminoacetamide hydrochloride, Glycine amide hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NH2CH2CONH2 · HCl
CAS Number:
Molecular Weight:
110.54
Beilstein:
3554199
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
204 °C (dec.) (lit.)
SMILES string
Cl.NCC(N)=O
InChI
1S/C2H6N2O.ClH/c3-1-2(4)5;/h1,3H2,(H2,4,5);1H
InChI key
WKNMKGVLOWGGOU-UHFFFAOYSA-N
Application
Buffer useful in the physiological pH range.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carolina García-Martínez et al.
The journal of pain : official journal of the American Pain Society, 7(10), 735-746 (2006-10-05)
Vanilloid receptor subunit 1 (TRPV1) is an integrator of physical and chemical stimuli in the peripheral nervous system. This receptor plays a key role in the pathophysiology of inflammatory pain. Thus, the identification of receptor antagonists with analgesic and anti-inflammatory
Lihu Yang et al.
Bioorganic & medicinal chemistry letters, 16(14), 3735-3739 (2006-05-16)
Systematic modification of a screening lead yielded a class of potent glycinamide based CCR2 antagonists. The best compound (55, (2S)-N-[3,5-bis(trifluoromethyl)benzyl]-2-{[2-(1-piperidinyl)ethyl]amino}-2-(3-thienyl)acetamide) displayed good binding affinity (IC50=30 and 39 nM) toward human monocytes and CHO cell expressing human CCR2b, respectively. Functionally, it
Hong Zhao et al.
Bioconjugate chemistry, 17(2), 341-351 (2006-03-16)
The utility of PEGylation for improving therapeutic protein pharmacology would be substantially expanded if the authentic protein drugs could be regenerated in vivo. Diminution of kinetic constants of both enzymes and protein ligands are commonly encountered following permanent bioconjugation with
Len Ito et al.
FEBS letters, 585(3), 555-560 (2011-01-18)
Glycine amide (GlyAd), a typically amidated amino acid, is a versatile additive that suppresses protein aggregation during refolding, heat treatment, and crystallization. In spite of its effectiveness, the exact mechanism by which GlyAd suppresses protein aggregation remains to be elucidated.
Yong Sun et al.
The journal of physical chemistry. B, 109(12), 5919-5926 (2006-07-21)
For the purpose of investigating the tautomerism from glycinamide (G) to glycinamidic acid (G*) induced by proton transfer, we carried out a study of structural interconversion of the two tautomers and the relative stabilizing influences of water during the tautomerization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service