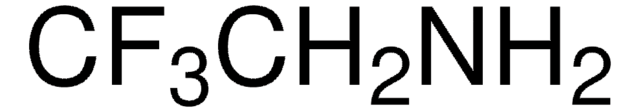549797
Acryloyl chloride
97.0%, contains <210 ppm MEHQ as stabilizer
Synonym(s):
2-Propenoyl chloride
About This Item
Recommended Products
vapor density
>1 (vs air)
vapor pressure
1.93 psi ( 20 °C)
Assay
97.0%
contains
<210 ppm MEHQ as stabilizer
refractive index
n20/D 1.435 (lit.)
bp
72-76 °C (lit.)
density
1.114 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
ClC(=O)C=C
InChI
1S/C3H3ClO/c1-2-3(4)5/h2H,1H2
InChI key
HFBMWMNUJJDEQZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Acrylic polymers via radical polymerization or copolymerization. These acrylic polymers can be tailored to possess the desired properties for biomedical coatings, including biocompatibility, adhesion to the device surface, and durability.
- Poly(styrene-co-acryloyl chloride) copolymer by crosslinked networks with styrene. The resulting crosslinked polymer can then be functionalized or modified by various chemical reactions to introduce specific properties or functionalities desired for the application as a polymer support or an electrophilic scavenger resin.
- Acrylamide-modified chitosan.
- Ulvan acrylate macromer via esterification of hydroxyl groups of polysaccharides. This macromer can be used to prepare ulvan-based thermosensitive hydrogels.
- Degradable peptide cross-linker by the acrylation of the amine groups of lysine residues and glutamine within peptide sequences.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Met. Corr. 1 - Skin Corr. 1A
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
30.2 °F
Flash Point(C)
-1 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service